
Human beings have always been fascinated by the brain.
Although scientific knowledge about this 1.3 kg of fragile substance embedded in our cranium has long been incomplete, dazzling technical breakthroughs made in recent years are now ushering in a Golden Age of molecular neuroscience.
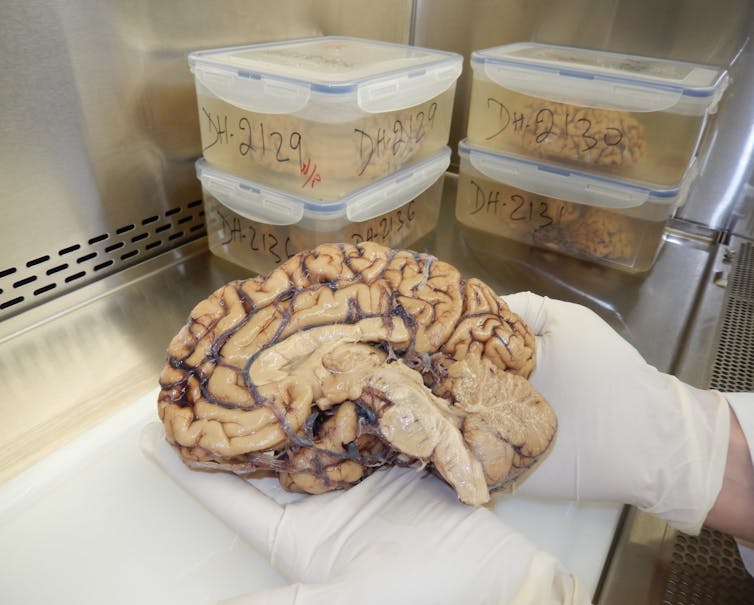
These breakthroughs have been made possible partly thanks to brain banks, which preserve human brains in the best possible conditions for scientific research. Here in Montréal, we have one of the world’s largest such banks, the Douglas-Bell Canada Brain Bank (DBCBB), founded in 1980 at the Douglas Hospital.
The DBCBB, which receives several brains each month, has collected over 3,600 specimens to date. Every year, its team processes dozens of tissue requests from scientists in Québec, Canada and abroad, preparing some 2,000 samples for research.
Over the past 40 years, these efforts have led to a considerable number of discoveries about different neurological and psychiatric diseases.
As a full professor in the department of psychiatry at McGill University, researcher at the Douglas Research Centre and director of the DBCBB since 2007, I work in close collaboration with Dr. Gustavo Turecki, co-director of the DBCBB and responsible for the component devoted to psychiatric illnesses and suicide.
A brief history of research on the human brain
Scientists only began to identify the microscopic elements that make up the human brain in the second half of the 19th century.
That was when brains were preserved for the first time in formalin, a solution that preserves biological tissue so that it can be handled more easily and stored over a longer term.
At the same time, precision instruments and protocols were being developed that made it possible to examine the microscopic characteristics of nervous tissue.
Until the middle of the 20th century, researchers were mainly satisfied with preserving the brains of patients, taken during autopsies, so they could use them to identify possible macroscopic or microscopic changes linked to either neurological or psychiatric symptoms.
This is in fact what the German neurologist Alois Alzheimer did when he analyzed the brain of one of his patients suffering from dementia. In 1906, he described, for the first time, the microscopic lesions which characterize the disease that now bears his name.
Until the end of the 1970s, numerous collections of brain specimens preserved in formalin were built in hospital environments, a bit like the cabinets of curiosities of olden days.
Towards the end of the 20th century, new experimental approaches were developed allowing the high-resolution analysis of cells and molecules within biological tissues.
It then became necessary to collect and preserve human brains, obtained with the consent of the individual or his or her family, in conditions compatible with modern scientific techniques.
Researchers began freezing one of the cerebral hemispheres in order to measure its various molecular components. The other hemisphere was preserved in formalin to be used for macroscopic and microscopic anatomical studies.
This was the context in which the Douglas-Bell Canada Brain Bank was created.

New experimental approaches are yielding results
Leading researchers from many universities around the world now use DBCBB samples to advance their research. This, of course, includes a number of teams in Québec.
For example, with his team from the Douglas Research Centre, which is affiliated with McGill University, Judes Poirier discovered that the APOE4 gene is a risk factor for Alzheimer’s disease. More recently, the team of Gilbert Bernier, professor in the department of neuroscience at Université de Montréal, discovered that the lesions characteristic of this disease are associated with abnormal expression of the BMI1 gene.
With regard to psychiatric illnesses, and more specifically depression, major progress has been made recently by the McGill Group for Suicide Studies.
Using cutting-edge methods to isolate and analyze human brain cells, Turecki’s team has succeeded in precisely identifying the cell types whose function is affected in men who have suffered from major depression, and then discovering that the cell types involved in this illness differ between men and women.
These experimental approaches generate huge data sets that can be examined in subsequent studies. This is the case, for example, of work carried out in my laboratory, which identified signs of persistent changes in neuroplasticity within the prefrontal cortex of people with a history of child abuse. In fact, the studies mentioned above enabled us to discover at least one of the cell types involved in this phenomenon.
In short, the experimental methods we have today allow us to break the brain down into its elementary components in order to understand its functions and dysfunctions.

Identify, prevent, screen and treat
Thanks to the hard work and dedication of the entire DBCBB team, as well as the unfailing support of all its partners, patrons (often anonymous) and funding bodies — particularly the FRQS research fund and Québec’s suicide research network, the Réseau québécois sur le suicide, les troubles de l'humeur et les troubles associés — this invaluable resource has not only managed to survive, but to grow and become one of the largest brain banks in the world.
There is every reason to believe that, in the years to come, the DBCBB will play an important role in the increasingly precise identification of the biological causes of brain diseases, and, as a result, will contribute to the identification of new targets for better approaches to prevention, screening and treatment.
Naguib Mechawar has received funding from CIHR, NSERC, HBHL (CFREF) and FQRS (NEURON ERA-NET and RQSHA).
This article was originally published on The Conversation. Read the original article.







