The U.S. Food and Drug Administration just approved the first new antibiotic to treat urinary tract infections in nearly three decades.
UTIs are infections in any part of the urinary system and affect as many as 16 million American women each year. One in four women are affected by a UTI in their lifetime, and approximately 30 percent are prone to painful recurring infections.
Blujepa, which is made by the British pharmaceutical company GSK plc, is approved for the treatment of women and children over the age of 12 with uncomplicated UTIs caused by bacteria including E. coli, Klebsiella pneumoniae, Citrobacter freundii complex, Enterococcus faecalis, and a type of staph infection known as Staphylococcus saprophyticus.
“The approval of Blujepa is a crucial milestone with uUTIs among the most common infections in women,” GSK’s Chief Scientific Officer Tony Wood said in a statement announcing the move.
“We are proud to have developed Blujepa, the first in a new class of oral antibiotics for uUTIs in nearly three decades, and to bring another option to patients given recurrent infections and rising rates of resistance to existing treatments,” he said.
The approval is based on results from two phase III clinical trials of 3,000 adults and teenagers. Phase III trials test if the treatment is better than what is currently available.
GSK said that the trials demonstrated “non-inferiority” to nitrofurantoin, which is an antibiotic that was approved to treat UTIs decades ago.
Blujepa was shown to successfully treat between 50 percent and 58 percent of infections when taken twice a day for five days. Those percentages are compared to 43 percent to 47 percent in participants that received nitrofurantoin.
There was one drug-related serious adverse event in both treatments across the two trials. In Blujepa, the most commonly reported adverse events were diarrhea and nausea.
A commercial launch of the antibiotic in the U.S. is planned during the second half of 2025.
GSK says that new treatments are needed as the number of UTIs caused by drug-resistant bacteria are increasing.
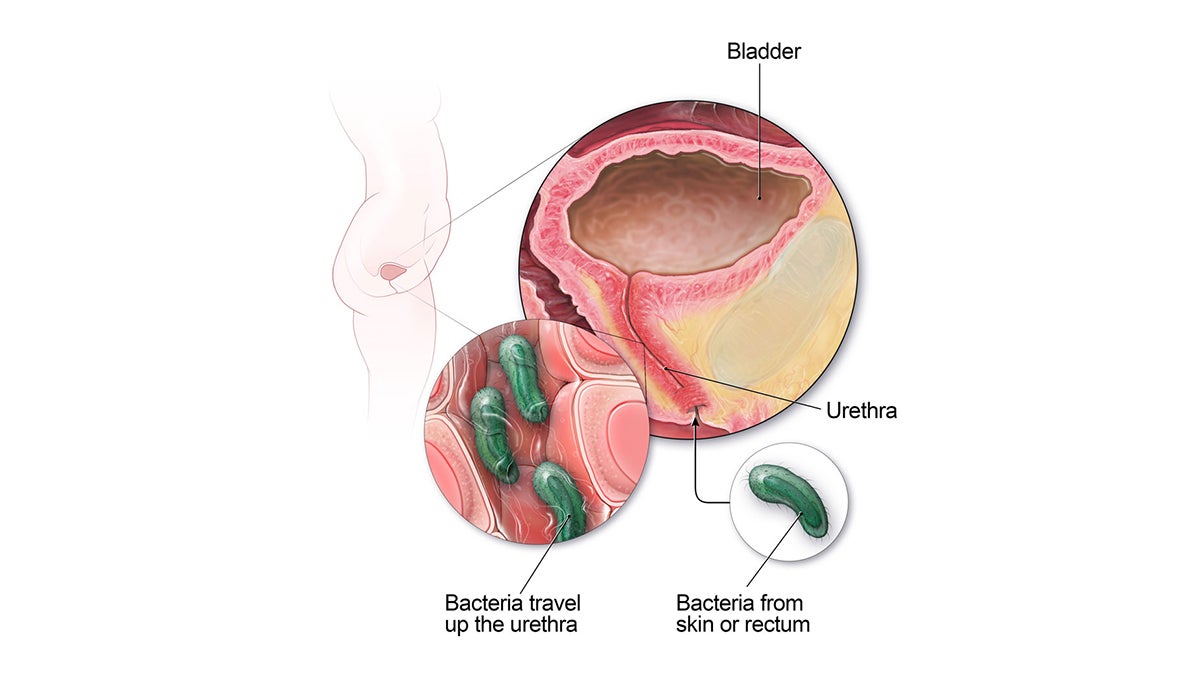
There are three main types of UTIs, affecting the urethra, bladder and kidneys. A bladder infection can result in pain while urinating, blood in urine, cramping and frequent urination. A kidney infection can lead to fever, chills, lower back pain and vomiting.
If a UTI is complicated, regular treatment with antibiotics isn’t enough to cure it. That can result in permanent kidney damage.
UTIs are more common in females because their urethras are closer to their rectum, making it easier for bacteria to enter the urinary tract. Risk factors change over time, with the most common factors before menopause including sexual intercourse and the use of chemical-based birth control. After menopause, Harvard Medical School says physical changes help set the stage for infections.
Women can prevent infections by urinating after having sex, staying hydrating and taking showers instead of baths.
“For many, UTIs can be a burden that severely impacts daily life,” Dr. Thomas Hooten, a professor of clinical medicine at the University of Miami School of Medicine, said. “With an increasing number of patients experiencing recurrent infections, there remains a clear need for continued research of antimicrobials to help address ongoing patient challenges and the strain on healthcare systems.”