
For thousands of years, humans have studied animals’ bodies to better understand our own. But using other animals as a proxy for humans is far from perfect. There are ethical issues for one, and they are never a perfect analog to the intricacies of the human body. In fact, when it comes to studying drugs, animal testing can lead to costly, time-consuming failures.
Nowadays, tech breakthroughs offer researchers new methods of modeling how the human body might react to cutting-edge treatments. And last December, a new U.S. law made it clear that these novel technologies are an option for testing drugs before moving on to human trials. Does that mean this is the end of animal testing?
A brief history of animal studies
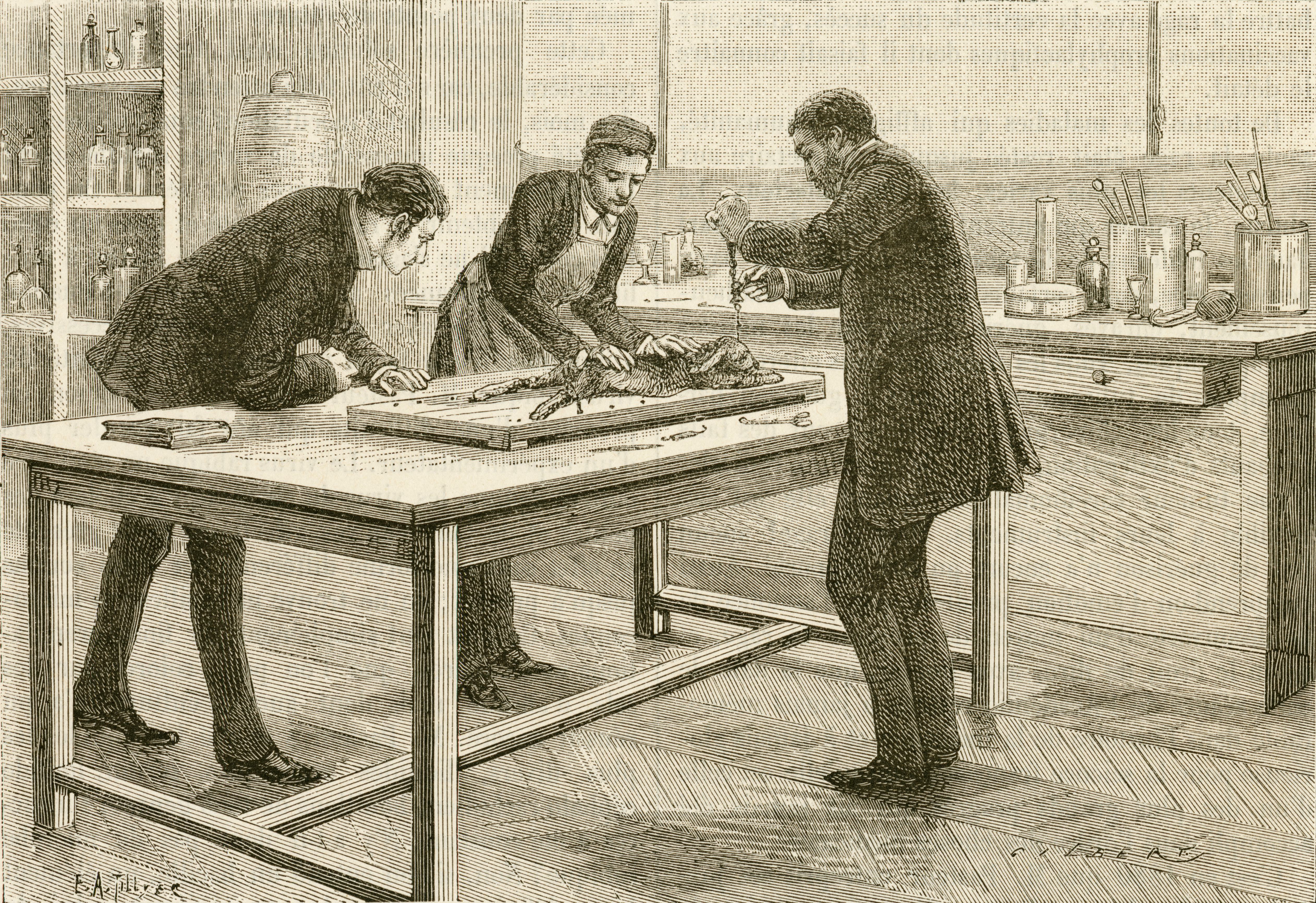
The earliest written records of people studying animals date back to the 4th century BCE, when Aristotle examined chicken eggs to learn how embryos developed (he and his fellow philosopher-physicians even dissected live animals). While social and religious mores forbade dissecting human cadavers, physicians continued cutting into critters for centuries.
As researchers worked on new medicines around the turn of the 20th century, animal models became a popular testing method. Scientists gave experimental treatments for diphtheria and diabetes to animals as proof of concept before sampling them in humans, earning Nobel Prizes.
The law remained unchanged for 84 years.
As drug production increased, it meant that a bad batch or a dangerous treatment could harm lots of people. For instance, a batch of diphtheria antitoxin infected with tetanus killed 13 children in 1901; in response, the U.S. government passed its first law regulating biological products in 1902. And when an antibiotic medicine containing a deadly poison killed more than 100 people in 1937, the government responded with the Federal Food, Drug, and Cosmetic Act of 1938. The law required manufacturers to run “preclinical tests (including tests on animals)” before medicines made it to human testing.
The agency never required animal testing across the board for new drugs, a spokesperson from the Food and Drug Administration told Inverse. But animal testing tended to be the only realistic means for companies to prove their drugs’ safety before human trials. The law remained unchanged for 84 years — until the recent adjustment, which enables companies to use novel testing methods developed from recent technological advancements.
The problem with furry subjects

Over the last few centuries, animal testing has pushed the boundaries of science and paved the way for drugs and vaccines that have saved countless lives.
Scientists use thousands of dogs — mostly beagles, chosen for their small size and friendly disposition — every year in animal testing. They’re typically euthanized for a more thorough screening of their organs after the testing is over. Mice, rats, and even monkeys are also common in drug testing. Some scientists argue that this suffering is largely in vain — animal models don’t even work incredibly well.
“I think for the whole time I've been in drug development, which has been a long time, everyone knows the animal models are terrible. The drug companies have always admitted it, the FDA has always admitted it,” Don Ingber, the founding director of Harvard University’s Wyss Institute for Biologically Inspired Engineering and co-founder of an organ-on-a-chip company called Emulate, tells Inverse.
Mice, rats, dogs, and monkeys are fundamentally different from humans. Different things make us sick, and when we get sick, our bodies respond in different ways, according to Ingber.
“Everyone knows the animal models are terrible.”
Scientists sometimes induce disease conditions in animals to study how that condition actually plays out, or to test how effective new drugs are at alleviating those symptoms. But “the disease models that they use often don't really replicate human diseases in a meaningful way,” Ingber says.
For instance, when scientists have tried to replicate sepsis in mice, the mice seemed to share symptoms with human patients, he says. But a closer look revealed key inconsistencies. “When people started analyzing the genes and molecules that mediate it, they're very different,” Ingber says.
These distinctions mean that drugs often make it through lengthy (and expensive) animal trials only to fail in human subjects — either because they don’t work as hoped, or because they cause harmful side effects. Nearly 95 percent of the drugs entering human trials fail, even after succeeding in animal trials, a 2019 study in the journal Translational Medicine Communication noted.
Despite these shortcomings, animal testing has remained the standard for drug development. “They had nothing better, and no one could offer an alternative,” Ingber says.
New technology could replace animal testing

In recent years, though, researchers have designed a whole slew of biomedical technologies to show precisely how human bodies may interact with new drugs.
Living human cells power many of these techniques. In 2006, Kyoto University professor Shinya Yamanaka announced that he’d found a way to coax adult mouse cells into a pluripotent state, capable of developing into a variety of different cell types. The research quickly led to the creation of human induced pluripotent stem cells, or iPSC.
Annelise Bennaceur-Griscelli, a professor at Paris-Saclay University in France, says this was a “wonderful” discovery because researchers no longer have to destroy a human embryo to source stem cells. Instead, they can take cells from a patient — for instance, via blood sample — and turn them into stem cells, which they can multiply in a lab setting and use to re-create various types of cells. What’s more, the stem cells reflect genetic alterations or chromosomal abnormalities associated with the patient’s illness.
These stem cells can be used in a variety of therapies. Right now, lots of scientists are using them to create organoids: 3D, hollow clusters of cells that mimic full-grown organs.
They’re tiny, but still visible to the naked eye. Bennaceur-Griscelli has worked on brain organoids that, after a year of growth, reached 2 to 3 millimeters in diameter. (A pea is about 10 millimeters.) They’re not full working models of, say, a brain or a heart, but they contain the relevant tissue types and rough versions of some of the structures in our organs.
These replicated organ tissues have proven useful for various tests and models, says Ali Turhan, a professor at Paris-Saclay University and one of Bennaceur-Griscelli’s collaborators. “Instead of doing a clinical trial using a drug which will require several years to recruit maybe thousands of patients, you can use your organoids,” he says. In addition to replacing or supplementing trials in human patients, organoids may reduce the need for animal models.
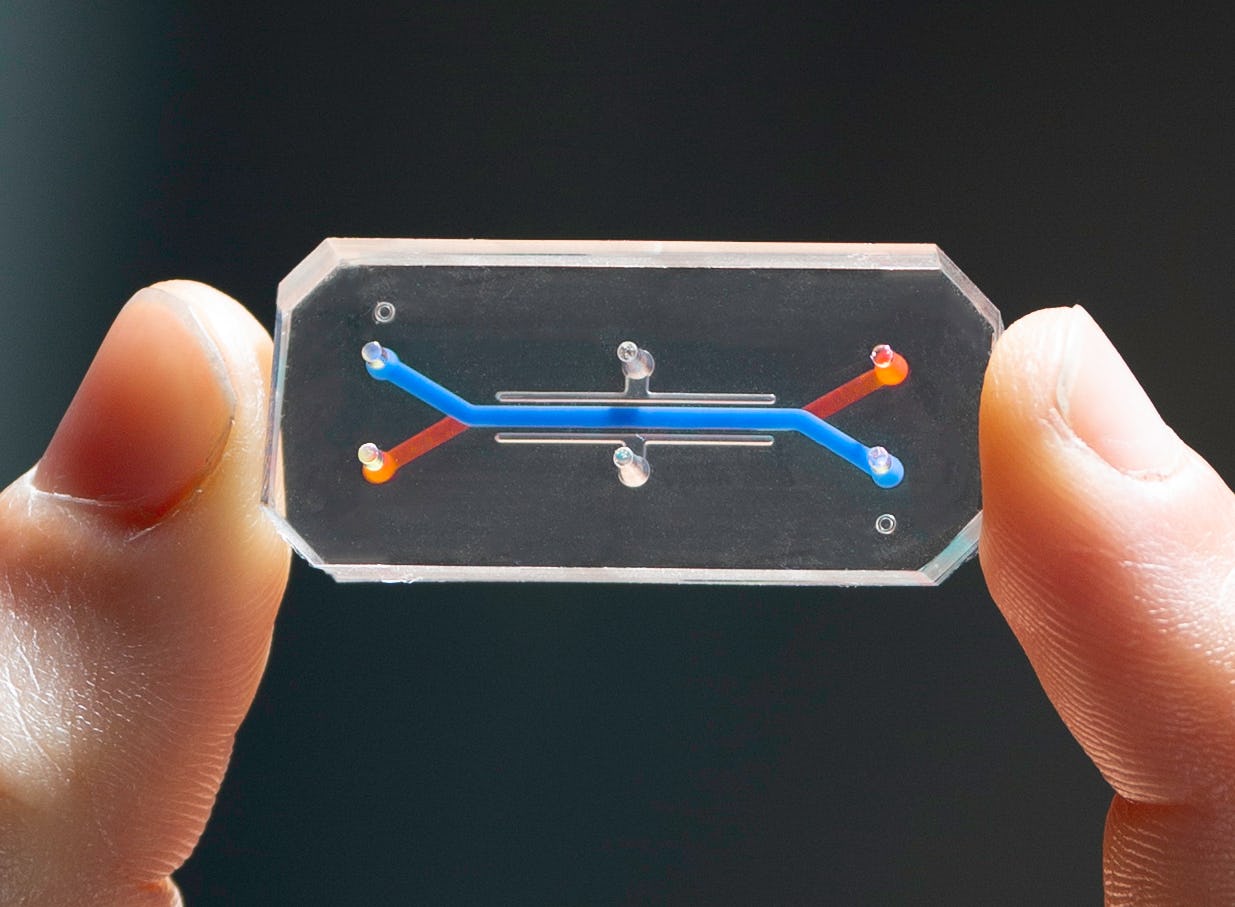
Human cells and tissues also make their way into fancy new devices called organs-on-chips. They’re around the size of a flash drive and contain narrow channels lined with living human cells.
“We can basically re-create the interfaces between tissues that make an organ,” says Ingber. “We have the blood vessel cells lining one channel and your lung cells in your air sac on the other. That's exactly how they interface in your body.”
Organs-on-chips can mimic the interactions between different kinds of tissues and the circulation of immune cells. Thanks to the channels’ flexible walls, they can even re-create the motions that go along with breathing and digestion.
“That's exactly how they interface in your body.”
When researchers introduce drugs into the chips’ microfluidic channels, the devices can simulate how different human tissues react to those drugs. Scientists can control the drug doses and monitor effects over time by moderating the flow of the drugs through the channels.
Some labs are bringing organs to the screen. Blanca Rodriguez, a professor of computational medicine at the University of Oxford, models drug interactions via computer simulations of the human heart.
“A lot of people have done experiments to measure processes that explain how the human heart works … we write mathematical equations that describe those data sets,” says Rodriguez. With data from heart cells’ interactions with a drug in a petri dish, researchers like Rodriguez can estimate that medication’s effects on an entire beating heart.
The end of animal testing?
These new technologies have already shown promise. Computer models have a higher success rate than animal models at predicting which drugs will cause heart problems in human patients, according to a 2017 paper by Rodriguez. She and her team have even suggested that scientists should work toward entirely replacing animal testing for heart drugs with computer simulations.
Ingber’s Emulate colleague Lorna Ewart tested livers-on-chips by exposing them to a group of drugs that had made it through animal testing but were found to cause liver damage in human trials, according to a 2022 study published in Nature. The chips correctly flagged 87 percent of the liver-toxic drugs that had sailed through animal trials.
These sorts of successes helped usher in an update to the 1938 Food, Drug, and Cosmetic Act. The revised law, which was signed by President Biden in December 2022, mentions cell-based assays, organs-on-chips, and computer modeling as viable models for gauging drug safety, alongside animal testing.
That doesn’t mean that animal testing will disappear overnight. Even the scientists who champion the new technologies note that in many cases, these new methods need to be supplemented with results from animal models, especially to determine a drug’s toxicity or see how all the organ systems interact with a new medication.
“There are still many areas where animal testing is scientifically necessary because the current state of science does not support replacement of all animal studies with non-animal methods,” Namandjé Bumpus, chief scientist at the FDA, tells Inverse. “Current non-animal methods cannot always predict effects that occur in such intricate systems.”
While Ingber agrees that a world without animal testing is a ways away, “in the near future, the hope is to progressively reduce the need for animal testing, one animal model at a time,” he says.
Scientific breakthroughs, coupled with the recent legislative change, may help convince more labs to explore non-animal testing options, which could make ripples throughout the pharmaceutical world.
“There are alternatives emerging,” Ingber says. “It just changes the mind frame.”







