
Penicillin was the world's first antibiotic, a type of medication that kills or slows the growth of bacteria.
After Scottish doctor Alexander Fleming discovered penicillin in a petri dish containing bacteria and mold, scientists eventually isolated the microbe-killing agent and used it to treat bacterial infections. Before mass-produced antibiotics, diseases such as pneumonia, tuberculosis and rheumatic fever were often deadly.
How does penicillin work?
Most bacteria have outer walls made of large molecules called peptidoglycan. Penicillin works by preventing bacteria from producing peptidoglycan, which causes their cell walls to weaken, collapse and spill the cells' innards. This kills the bacteria.
Some bacteria produce beta-lactamase, an enzyme that can damage penicillin and block its effects. To stop this from happening, penicillins may be combined with substances that can neutralize beta-lactamase, such as clavulanic acid, according to the medical resource StatPearls.
What does penicillin treat?
Today, natural and semisynthetic types of penicillin are widely used around the world to treat a range of bacterial infections, including pneumonia, strep throat, bacterial meningitis and certain sexually transmitted bacterial infections, such as syphilis, according to the National Institute for Health and Care Excellence. It is a well-researched antibiotic with few side effects.
However, the overuse of penicillin and other antibiotics has driven some strains of bacteria to develop resistance to these drugs, making bacterial infections more difficult, and sometimes impossible, to treat.

How are penicillins made?
Penicillins can be divided into two groups: natural and semisynthetic. Natural penicillins are made through the fermentation of the fungus Penicillium chrysogenum, which produces the antibiotic compound as it grows. Semisynthetic penicillins are typically produced in a laboratory from a penicillin-derived substance called (+)-6-aminopenicillanic acid (6-APA), according to the Encyclopedia of Toxicology, Third Edition (Academic Press, 2014).
How is penicillin administered?
Penicillins are often administered through an injection, into either a vein or a muscle, in divided doses several hours apart. Some types of penicillin, such as penicillin V, can also be taken orally as liquid or tablets. The route of administration — through a needle or ingestion — affects how the drug is absorbed by the body and how much reaches the target bacteria, according to StatPearls.
Is penicillin the same as amoxicillin?
Amoxicillin is a widely-used penicillin derivative created by adding an extra chemical group to penicillin. It is administered orally as it is more resistant to the effects of stomach acids than most other penicillins and better absorbed in the digestive system, according to a 2009 review published in the journal Infectious Disease Clinics of North America. It also kills a wider spectrum of bacteria than penicillin.
What are the side effects of penicillin?
Penicillin is relatively safe, although a small percentage of people are allergic to the antibiotic. In people who are not allergic, penicillin still carries a small risk of side effects, such as upset stomach, nausea, vomiting and diarrhea, and a flat, red rash that resolves on its own, according to the medical resources UpToDate and StatPearls.
Specifically, penicillin G can cause electrolyte imbalances, particularly when given in large doses, and may cause effects like muscle spasms and pain, fever or low blood pressure.
What happens when someone is allergic to penicillin?
In penicillin allergy, the immune system mistakenly reacts to penicillin as if it were a harmful substance and releases compounds like histamines into the bloodstream, according to the American Academy of Allergy, Asthma & Immunology (AAAAI).
These compounds cause hives (a raised, itchy rash) and swelling around the face, hands and feet. Doctors typically treat penicillin allergy with antihistamines and sometimes corticosteroids. Rarely, people can have a life-threatening reaction to penicillin called anaphylaxis, which requires immediate treatment with epinephrine, the hormone in EpiPens. Further treatments may include albuterol to relax and open the airways, IV fluids and corticosteroids.
An allergic reaction to penicillin typically occurs less than an hour after someone receives a dose of the antibiotic, according to the AAAAI.
Around 10% of the U.S. population report having a penicillin allergy, but rough estimates suggest that less than 1% of the population may be truly allergic to this class of antibiotic, according to the CDC. And the CDC notes that 80% of people with a valid diagnosis lose their sensitivity to penicillin within 10 years.
Doctors can confirm a penicillin allergy using a skin prick test, during which a small amount of the antibiotic is injected into the skin. If an itchy bump appears within 30 minutes of the test, the patient is likely allergic to penicillin. Individuals who test positive may be prescribed a different antibiotic medication, according to Yale Medicine.
However, if penicillin is absolutely necessary — for example, when a life-threatening infection has no therapeutic alternatives — a patient may need drug desensitization treatment. This involves administering progressively greater doses of penicillin every 15 to 20 minutes until a full therapeutic dose is reached, allowing the immune system to temporarily tolerate the drug.

How was penicillin discovered?
Scottish physician and bacteriologist Alexander Fleming accidentally discovered penicillin in his lab in 1928.
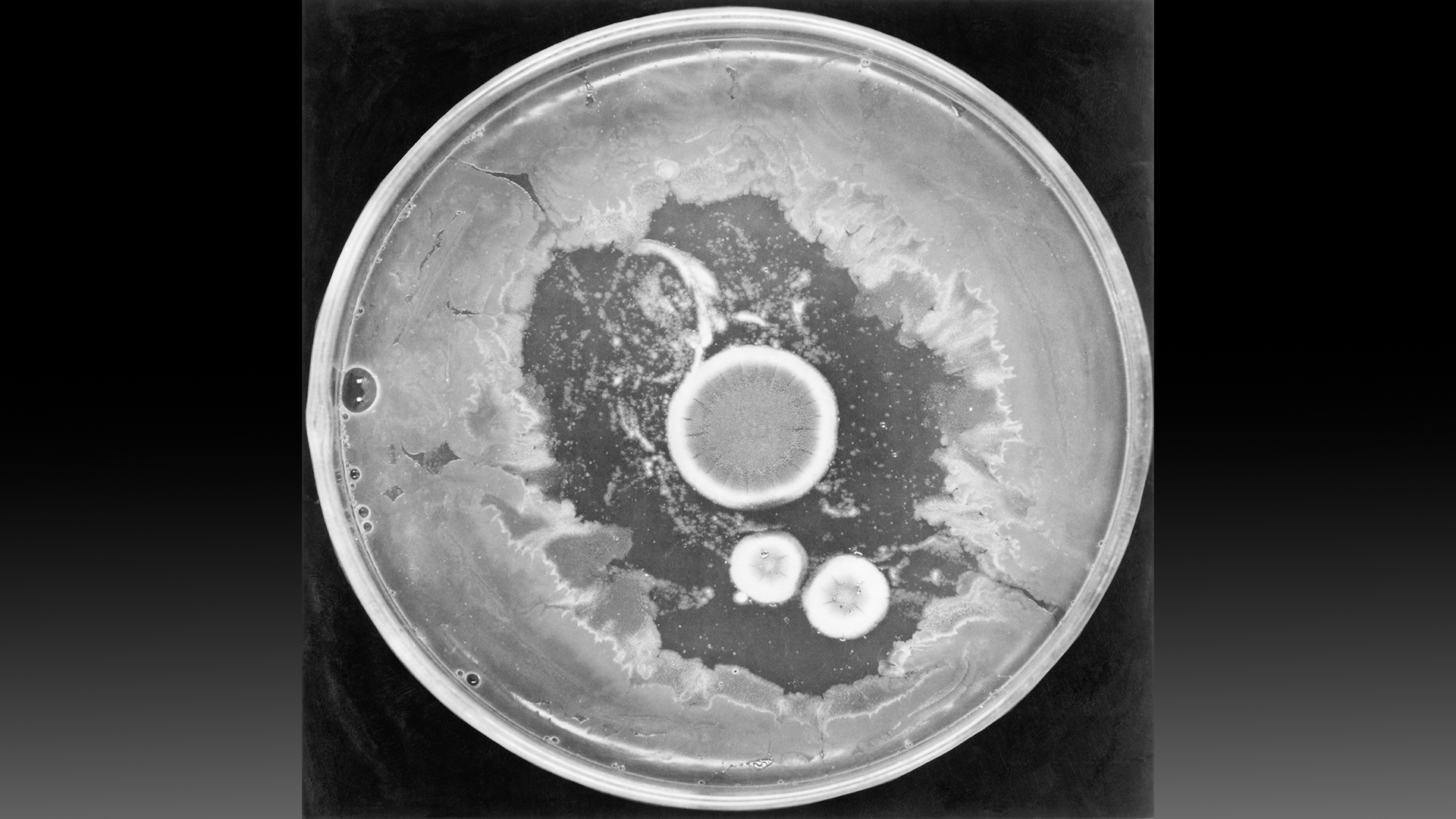
After returning from vacation, he noticed some petri dishes containing Staphylococcus bacteria had been contaminated with a mold in the Penicillium genus. The Staphylococcus did not grow as expected in spots that the fungus invaded. Fleming obtained an extract from the mold, named its active agent "penicillin" and determined that the extract killed several types of harmful bacteria, according to a 2017 article published in the journal Emerging Infectious Diseases.
Fleming published his findings in 1929, but he could never isolate the newfound compound. For a decade, Fleming sent his Penicillium mold to anyone who requested it, in hopes that they might be able to obtain pure penicillin, to no avail.
The compound was finally isolated in 1939 by a group of scientists led by Ernst Chain and Howard Florey, biochemists at the University of Oxford in England. They also conducted the first test of penicillin on animals, injecting eight mice with harmful bacteria and then providing four of the rodents treatment. The next day, all of the untreated mice had died, but the treated animals survived.
On Feb. 12, 1941, Florey's team gave the first dose of penicillin to a human, according to the American Chemical Society (ACS). Albert Alexander had a life-threatening infection, and within days of receiving penicillin, he began to recover. Unfortunately, Florey's team ran out of the drug before Alexander was completely healed, and he died.
According to the Emerging Infectious Diseases article, in June 1941, Florey and Chain traveled to meet Charles Thom, the principal mycologist with the U.S. Department of Agriculture, and Andrew Jackson Moyer, director of the department's Northern Research Laboratory.
Thom identified the species of mold that enabled Fleming's initial discovery — Penicillium notatum — which had initially been classified as P. rubrum. He also helped determine that a different Penicillium species, P. chrysogenum, produced six times more penicillin than Fleming's strain. Moyer suggested using a waste product of cornstarch manufacturing to grow the mold and make copious penicillin, and soon after, drug companies developed a new fermentation technique to do the same at industrial scales.
Production ramped up and in 1945, Fleming, Florey and Chain received the Nobel Prize in physiology or medicine "for the discovery of penicillin and its curative effect in various infectious diseases."
How does penicillin contribute to antibiotic resistance?
The misuse of penicillin contributes to the development of antibiotic resistance.
Antibiotics kill bacteria that are sensitive to the drug while drug-resistant bacteria strains grow and multiply. Being repeatedly exposed to antibiotics pressures bacteria to evolve new strategies to resist the drugs, and they can then share those strategies with other bacteria through a process called "horizontal gene transfer," according to the Centers for Disease Control and Prevention (CDC).
The spread of resistance to penicillin was first documented in 1942 in multiple strains of Staphylococcus aureus, which cause many skin and respiratory infections. Penicillin resistance has since emerged in other pathogens, including S. pneumoniae and Escherichia coli, according to a 2017 review published in The Yale Journal of Biology and Medicine.
Antibiotics should not be prescribed for viral infections, such as colds, influenza, most sore throats and bronchitis, the CDC states. That's because people carry penicillin-sensitive bacteria in their body all the time without the bugs causing disease. When doctors give penicillin for viral infections, it does nothing to treat the illness but does pressure the penicillin-sensitive bacteria harmlessly living in the body to evolve resistance.
According to the CDC, at least 28% of antibiotics prescribed in outpatient settings are not needed by the patients, and up to half of all antibiotic use in these clinics may be inappropriate due to doctors selecting the wrong antibiotic, dosage or treatment duration.
"Overall, there is a major problem with inappropriate antibiotic prescribing in the United States," Dr. Saul R. Hymes, medical director for pediatric antimicrobial stewardship at Stony Brook Children's Hospital in New York told Live Science.
This article is for informational purposes only and is not meant to offer medical advice.
Additional resources
To learn more about the serendipitous discovery of penicillin, check out this TED-Ed talk. Watch this SciShow video for a digestible explanation of what antibiotic resistance is and how it spreads. And for more guidance on how antibiotics should (and should not) be used, go to the CDC's "Antibiotic Do's & Don'ts" page.







