Following the announcement of a possible link between the AstraZeneca vaccine and rare blood clots, the European Medicines Agency (EMA) is now investigating whether the vaccine is linked to a second very rare blood disorder: systemic capillary leak syndrome (SCLS).
To date, five cases of SCLS in recently vaccinated people have been reported to the EMA. The regulator’s Pharmacovigilance Risk Assessment Committee is now working to see if a causal link with the AstraZeneca vaccine can be established – as it did following reports of blood clots – and whether new safety guidelines for the vaccine are needed.
This investigation is a response to what the EMA calls a “safety signal”. These are unusual events that have been flagged up as possible side-effects of a given medicine, but which need researching in-depth for this to be confirmed.
As the EMA notes, receiving safety signals doesn’t itself prove that a medicine causes the adverse events being reported – illness or other medicines could also be the cause. This is why every case needs to be thoroughly investigated, and we should avoid jumping to conclusions.
However, with tens of millions of vaccines having been given, it’s very difficult to tease out whether such rare events can be attributed to the vaccine or are appearing as part of the normal course of events in a population.
A mysterious condition
We also don’t know a lot about SCLS in the first place. There have been fewer than 500 cases reported since it was first described by researchers in 1960 (it is also known as Clarkson’s disease, after one of these scientists).
In this disorder, the blood plasma, the yellowish liquid part of the blood, leaks out from the smallest blood vessels, the capillaries, into the surrounding tissues, potentially causing great damage. It can lead to organ failure and death if untreated.
Sufferers have irregular episodes that are different in their intensity depending on the individual. This means that making a diagnosis can be difficult. Sufferers may also feel tired and have water retention (also known as oedema, where fluid builds up in the body), both of which are relatively common – particularly in the elderly – and may be caused by many things. This can delay people reporting such symptoms to their GP.
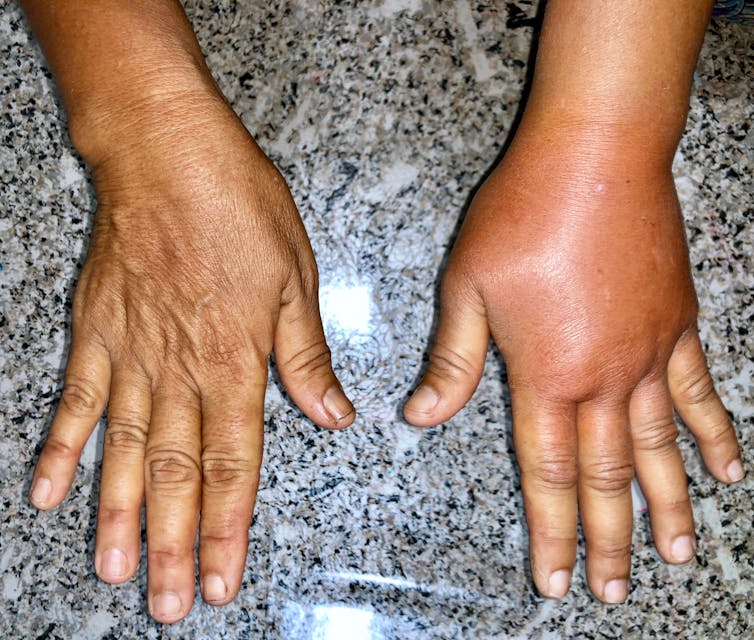
Unsurprisingly, SCLS can be misdiagnosed. Its symptoms are similar to those seen in sepsis, which also causes the capillaries to leak. SCLS also causes low blood pressure, low levels of albumin (a protein found in the blood plasma) and a high red blood cell concentration – all signs of other, more common disorders.
Together, these factors may have led to cases of the condition being missed in the past, which in turn may have hampered efforts to understand it better. It’s also plausible that they might make it harder for people to spot instances of SCLS now. However, this may be counterbalanced by the fact that people are now highly alert to the possibility of vaccine side-effects, and so may be more vigilant in picking them up – a recognised phenomenon known as “notoriety bias”.
There’s also a common feature of SCLS that could be useful for identification: the production of an abnormal immune protein, known as M protein. This occurs in up to 85% of patients who are diagnosed with the condition. However, the production of M protein also occurs in some types of blood cancer, such as myeloma, a much more common disease among the elderly (who have predominantly taken the vaccine).
What do we know about its causes?
Studies show that during SCLS there seem to be changes in the integrity of blood vessel walls, accompanied by a disordered immune response. It’s possible therefore that the immune system is directly affecting the blood vessels; we know from another condition – vasculitis – that an autoimmune reaction to the blood vessels can happen.
Patients who have been diagnosed with SCLS tend to be carefully given fluids to manage their symptoms, and some patients have improved after being given intravenous immunoglobulin (the protein from which antibodies are made). This seems to prevent further episodes, highlighting the immune system’s likely role in the disease. But the specific causes of SCLS are unknown.
The EMA will be looking closely at whether a possible mechanism is the AstraZeneca vaccine causing the immune system to attack blood vessels. A single similar incidence of this was reported in 2015, when a dialysis patient developed SCLS after an influenza vaccine. Although researchers were unable to link this occurrence of SCLS to the vaccine, they nevertheless reported it to the Netherlands Pharmacovigilance Centre as a possible side-effect.
Another question for the EMA is whether this rare blood disorder can be linked to the other recently reported safety signal: blood clots with low platelet counts. While it may be tempting to link the two conditions with each other and the vaccine rollout, there’s no evidence yet to suggest they are connected. Even if both disorders are proven to be linked to the vaccine, they could still be completely unconnected.
It’s important to remember that there have been very few cases of this syndrome reported so far. If the regulators do establish that there’s a causal relationship between SCLS and the vaccine, the benefits of taking it still need to be weighed correctly against the risks, with this being reflected in any future guidance and communications.
Peter Abel does not work for, consult, own shares in or receive funding from any company or organization that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.
This article was originally published on The Conversation. Read the original article.







