The human metabolic system is a marvel of biological engineering, involving thousands of genes that interact in intricate pathways to sustain life. Disruptions in these pathways often underlie chronic diseases, such as diabetes, obesity, and metabolic syndromes. To better understand these processes and develop targeted treatments, scientists have increasingly turned to human metabolic gene knockout libraries as a revolutionary tool. This technology allows for high-throughput exploration of gene function, paving the way for groundbreaking discoveries in human metabolism and disease.
What Is a Human Metabolic Gene Knockout Library?
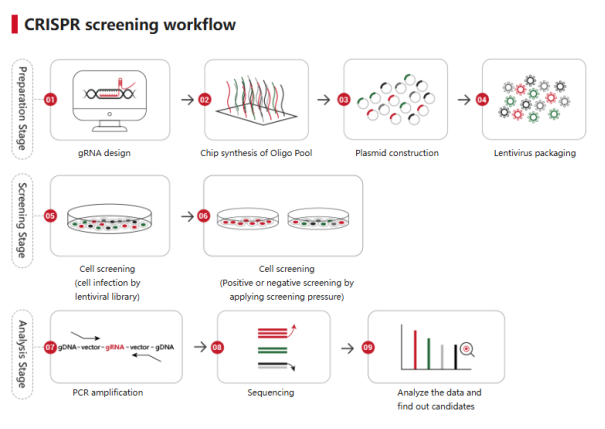
A human metabolic gene knockout library is a specialized collection of tools that use CRISPR-Cas9 technology to systematically inactivate genes associated with human metabolism. Each gene in this library is targeted by a single-guide RNA (sgRNA), enabling researchers to "knock out" specific genes in metabolic pathways.
These libraries are designed to:
1. Investigate Gene Function: Identify the roles of individual genes in metabolic pathways.
2. Study Disease Mechanisms: Explore how metabolic dysfunction arises from gene disruptions.
3. Accelerate Drug Development: Discover novel therapeutic targets for metabolic disorders.
Unlike traditional methods, such as RNA interference (RNAi), which suppresses gene expression at the RNA level, CRISPR-based knockout libraries directly modify the genome, providing permanent and more accurate results.

Applications in Metabolic Research
The potential of human metabolic gene knockout libraries extends across a wide range of research areas, making them indispensable for unraveling the complexities of human metabolism.
1. Disease Modeling
By knocking out specific metabolic genes, researchers can create precise models of human diseases, such as type 2 diabetes and fatty liver disease. These models help identify the key genetic players driving disease progression and offer insights into potential interventions.
2. Drug Target Discovery
Metabolic diseases often result from dysregulated pathways. By systematically knocking out genes, scientists can identify critical nodes within these pathways, revealing novel drug targets. For instance, genes that regulate glucose metabolism or lipid homeostasis could serve as key therapeutic entry points.
3. Functional Genomics
Understanding how metabolic genes interact requires a systems biology approach. Knockout libraries enable researchers to dissect these interactions, revealing how genetic networks contribute to metabolic stability or dysfunction.
4. Precision Medicine
By tailoring knockout experiments to specific genetic backgrounds, researchers can uncover why certain individuals are more susceptible to metabolic disorders. This personalized approach is essential for developing targeted therapies and advancing precision medicine.
Key Advantages of Using Knockout Libraries
Human metabolic gene knockout libraries bring several advantages over traditional research methods, including:
1. High Precision: CRISPR-Cas9 technology ensures highly specific gene editing, reducing off-target effects and increasing the reliability of results.
2. Comprehensive Coverage: These libraries are designed to target all known metabolic genes, enabling systematic studies of the entire metabolic genome.
3. Scalability: Whether exploring a single gene or conducting genome-wide screens, knockout libraries are versatile and scalable for diverse research needs.
4. Efficient Drug Development: By identifying and validating potential drug targets, knockout libraries streamline the drug discovery pipeline, saving time and resources.
Challenges and How to Overcome Them
Despite their transformative potential, human metabolic gene knockout libraries are not without challenges. Addressing these issues is key to fully leveraging their capabilities:
1. Maintaining Experimental Integrity
Large-scale knockout experiments require careful design and execution to ensure reproducibility. Using high-quality libraries and optimized protocols can minimize variability.
2. Off-Target Effects
Although CRISPR-Cas9 is highly specific, off-target effects may still occur. Advanced sgRNA design algorithms and high-fidelity Cas9 variants can mitigate these risks.
3. Complex Data Analysis
Knockout studies generate massive amounts of data, requiring robust bioinformatics tools for interpretation. Collaborative efforts between biologists and computational scientists are essential for deriving meaningful insights.
The Future of Metabolic Research with Knockout Libraries
The field of metabolic research is evolving rapidly, and human metabolic gene knockout libraries are at the forefront of this transformation. Here are some promising trends:
1. Integration with Multi-Omics: Combining knockout libraries with transcriptomics, proteomics, and metabolomics will provide a holistic view of metabolic regulation.
2. Single-Cell Applications: Single-cell CRISPR screening allows researchers to study metabolic gene function at an unprecedented level of detail, revealing cell-to-cell variability.
3. Therapeutic Development: As we gain a deeper understanding of metabolic gene networks, knockout libraries could inform the development of gene-editing therapies for metabolic diseases.
4. Machine Learning Integration: Advanced computational tools can predict gene functions and interactions, guiding the design of more efficient knockout experiments.
Conclusion
Human metabolic gene knockout libraries have emerged as a revolutionary tool in metabolic research. By enabling precise and systematic investigation of gene function, these libraries are driving breakthroughs in understanding metabolic diseases and uncovering novel therapeutic targets. As technology continues to advance, the integration of knockout libraries with cutting-edge methodologies promises to reshape our understanding of human metabolism and pave the way for innovative treatments.
With their unmatched precision and versatility, these libraries are more than just tools—they are the key to unlocking the secrets of human metabolism and addressing some of the most pressing health challenges of our time. For researchers seeking to make impactful discoveries, there has never been a better time to explore the potential of human metabolic gene knockout libraries.







