Over the past 40 years, changes in our urban environment and diet have had a major impact on our lifestyles.
We are more sedentary and the quality of our diet and sleep is at its lowest in decades. These changes, coupled with an increase in life expectancy, are associated with an increase in the number of people with “cardiometabolic” diseases such as Type 2 diabetes, heart diseases, certain cancers and even certain neurodegenerative diseases.
Another cardiometabolic disease that frequently flies under the radar is non-alcoholic fatty liver disease. The liver is an important organ for food digestion, energy metabolism and nutrient management, and communicates with the intestine and the adipose tissue (the main component of our body fat). But non-alcoholic fatty liver disease is a fairly silent disease, as there are few or no symptoms associated with it.
Our lab uses human genetics to identify targets to treat and prevent non-alcoholic fatty liver disease and its complications.
Fatty liver disease and its consequences
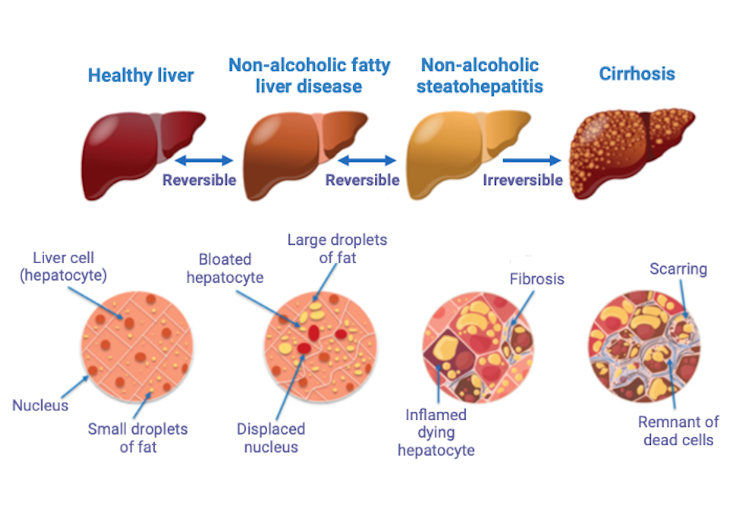
Non-alcoholic fatty liver disease is a disease that affects, on average, one in four adults and nearly one in 10 children worldwide. The disease progresses from reversible to irreversible stages.
The first stage is defined by the presence of steatosis (excessive accumulation of lipids in the liver to at least five per cent of the total liver mass). The next stage, which is also reversible, is characterized by inflammation of the liver cells (called hepatocytes). This inflammation may be accompanied by scar tissue (called fibrosis).
The development of the disease to irreversible stages, in more severe cases, can lead to cirrhosis and/or liver cancer. By 2025, non-alcoholic fatty liver disease will be the leading cause of liver failure and transplantation. Its complications, however, are not limited to liver disease. It is strongly associated with several other cardiometabolic diseases such as Type 2 diabetes and cardiovascular diseases (the leading cause of death of those with non-alcoholic fatty liver disease).
What are the risk factors?
Non-alcoholic fatty liver disease develops gradually and may progress differently from one individual to another depending on genetic factors and certain risk factors, including diet.
Consuming added sugar, such as fructose in sweetened beverages, may contribute to its development, by activating a metabolic process called “de novo lipogenesis”, the production of fatty acids from sugar. Ultra-processed products, common in the North American diet, have a high energy density and provide a high intake of sugar, fat and salt. Furthermore, alcohol consumption, even in the absence of alcoholism, could have a synergistic effect on liver metabolism and accelerate the progression of non-alcoholic fatty liver disease.
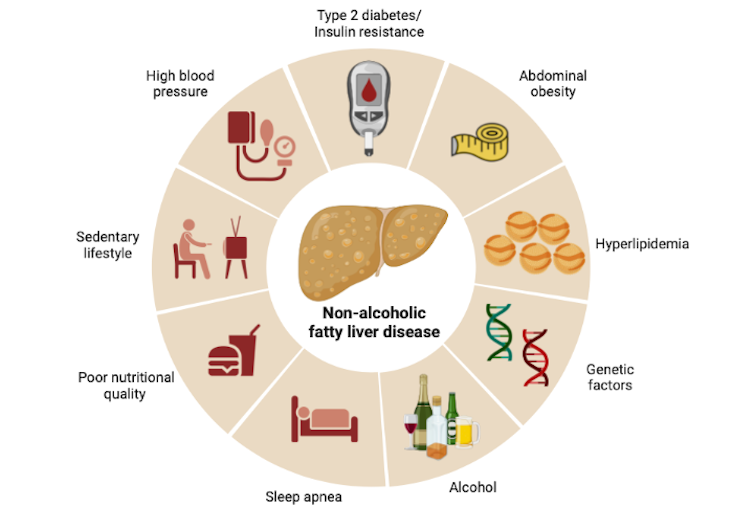
Being overweight is also risk factor for non-alcoholic fatty liver disease: About half of obese individuals (people with a body mass index (BMI) greater than or equal to 30) could develop the disease. However, non-alcoholic fatty liver disease is frequently observed in people who have a “healthy weight.” Although BMI may have some utility in measuring the consequences of high body weight, its clinical utility is increasingly being questioned. BMI gives little or no information on the location of fat tissue: fat has much more harmful consequences when located in the abdomen than in the arms, hips or thighs.
Genetic factors
Our research team believes that identifying the genetic factors that contribute to chronic diseases such as non-alcoholic fatty liver disease will allow us to understand, prevent and treat them better.
To this end, we have conducted the largest genetic study of this disease to date. We compared the genome variations of 8,434 people with non-alcoholic fatty liver disease from four countries (Estonia, United States, Finland and the United Kingdom) with those of 770,180 people without the disease, and identified several susceptibility genes, including an association between a gene called LPL and non-alcoholic fatty liver disease. This gene, which codes for an enzyme called lipoprotein lipase, plays an important role in the storage of blood lipids in our adipose tissue. A disruption in the activity of the LPL gene could increase the chances of lipids being deposited elsewhere in the body, such as in the liver.
This genetic study also allowed us to clarify the role of distribution or localization of adipose tissue and obesity in the development of non-alcoholic fatty liver disease. In a recent study, which is currently under peer-review, we investigated the contribution of BMI and waist circumference to the presence of non-alcoholic fatty liver disease. A larger waist circumference was strongly associated with having a greater risk of developing non-alcoholic fatty liver disease, independent of BMI. Conversely, BMI alone had no effect on risk after considering waist circumference.
So, is it necessary to lose weight in order to prevent fatty liver disease?
Prevention or a cure?
Although some the medications used to treat Type 2 diabetes could reduce inflammation in the liver of patients with non-alcoholic fatty liver disease, there is no specific treatment or recommended supplements for the disease at this time.
We believe that identifying the genes implicated in non-alcoholic fatty liver disease will accelerate its treatment. Until then, targeting risk factors associated with non-alcoholic fatty liver disease seems to be the most promising avenue. Interestingly, several studies have shown that improving nutrition and increasing physical activity levels can reduce liver fat accumulation, although these factors were associated with relatively modest weight loss.
Like other societal chronic diseases such as cardiovascular diseases and Type 2 diabetes, fatty liver disease can be prevented to some degree. Daily activity, cooking a good variety of foods, improving sleep and limiting screen time, the consumption of ultra-processed products and exposure to stress, can prevent or delay the development of such diseases.
We believe that by democratizing access to a healthy diet and transforming urban planning to promote active travel, it will be possible to slow down the progression of such diseases in the population as a whole.
Benoit Arsenault receives funding from CIHR and Fondation de l'Institut Universitaire de cardiologie et de pneumologie de Québec.
Émilie Gobeil receives funding from Fonds de recherche du Québec - Santé.
This article was originally published on The Conversation. Read the original article.