
It may not come as a surprise to Apple fanatics or PlayStation devotees who scramble for the latest gadget, but electronics now make up the fastest growing kind of waste in the world. To help reduce our e-waste, scientists have developed a novel battery — one that’s disposable and made of paper, as detailed in a new Scientific Reports study. And while getting paper wet often renders it useless (unless you’re into paper mache), this new device is activated by water.
HERE'S THE BACKGROUND — To cut down on tech rubbish, scientists have previously investigated ways to make a variety of devices biodegradable, such as solar cells. However, work on biodegradable batteries is scarce because battery research mostly focuses on improving performance.
In the new study, researchers focused on abundant, cheap biodegradable materials such as the cellulose that composes wood and paper, as well as common mineral elements like carbon and zinc. They also employed simple manufacturing techniques such as printing to make it easy to scale up production.
"What we wanted to achieve with this specific work was to use sustainable materials and processing as a starting point to drive the development of creating environmentally safely disposable batteries," study senior author Gustav Nyström and lead of the laboratory for cellulose and wood materials at the Swiss Federal Laboratories for Materials Science and Technology in Dübendorf, Switzerland, tells Inverse.
WHAT DID THE SCIENTISTS DO? — The basic component of the new battery is a centimeter-wide square cell consisting of three inks printed onto a strip of paper. Sodium chloride (that is, table salt) is dispersed throughout the paper. One end of the strip is dipped in wax.
An ink containing flakes of graphite — the material that makes up pencil lead — acts as the cathode, or positive end of the battery, and is printed onto one side of the cell. Another ink containing zinc powder acts as the anode, or negative end of the battery, and is printed on the cell's other side. The last ink, containing graphite flakes and carbon black, is printed on both sides of the paper on top of the other two inks and connects the cathode and anode to two wires located at the paper’s wax-dipped end.
Adding water dissolves the salt in the paper, breaking it apart into electrically charged ions. These ions help electricity flow between the anode and cathode, generating an electric current that can power devices.
WHAT DID THEY FIND? — Experiments with a single-cell battery revealed that after two drops of water were added, it activated within 20 seconds, reaching a stable voltage of 1.2 volts. In comparison, a standard AA alkaline battery has a voltage of 1.5 volts.
After one hour, the single-cell battery's performance dropped significantly because the paper dried, but after two drops of water were added, it maintained a stable operating voltage of 0.5 volts for over one additional hour.
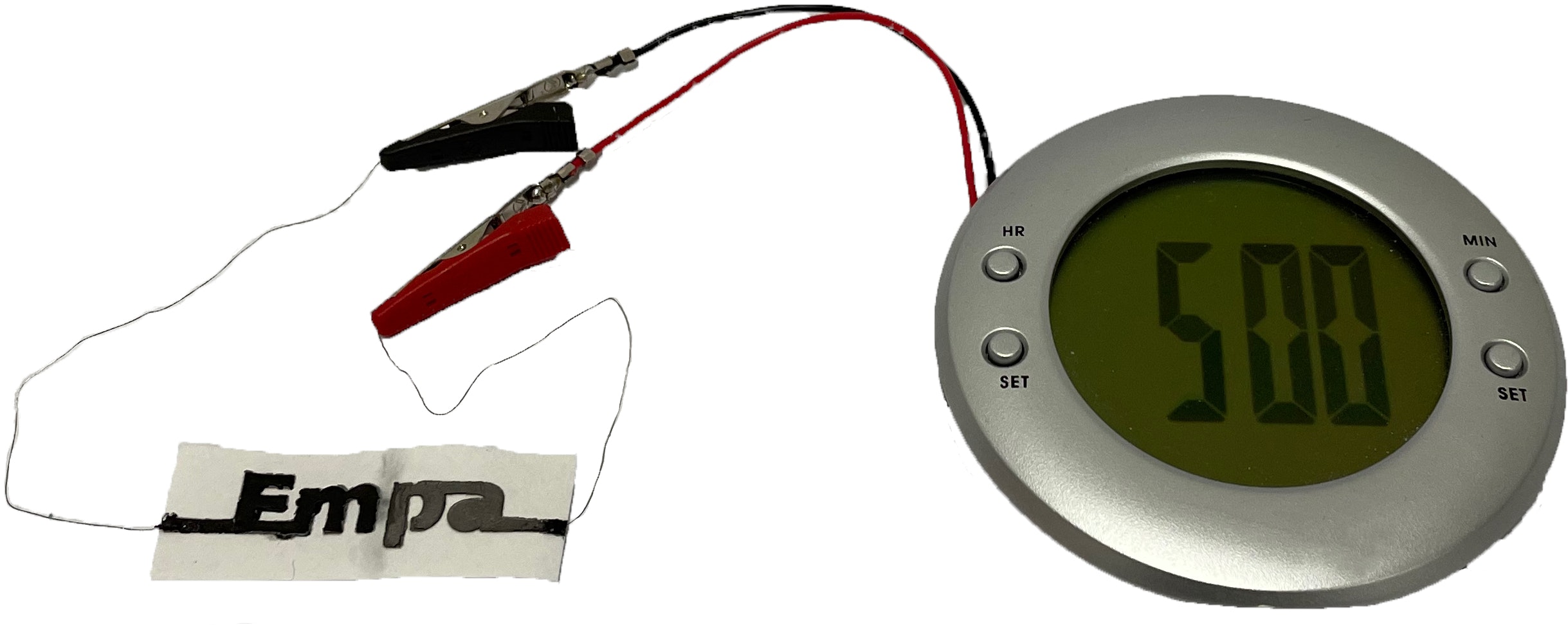
Nyström cautions that "this is not battery technology to replace high-power or consumer electronic devices. These materials and devices will never and were never meant to power, for example, smartphones or electric vehicles." Still, in tests, a battery with two cells could power an alarm clock with an LCD display.

WHAT'S NEXT? — The researchers suggest their paper batteries could help reduce the environmental impact of single-use low-power disposable electronics. For example, medical diagnostic devices, smart labels used to help track packages, and sensors to monitor food or the environment.
“We are already working on ways to further improve the energy and power densities of these batteries, while still being environmentally friendly,” Nyström says. “We are also working on platforms where the batteries are integrated together with other components such as sensors.”
Along with Nyström’s team, researchers around the world are tinkering with paper batteries. For example, Hongjin Fan and his colleagues at Nanyang Technological University in Singapore developed a paper battery last year with manganese-zinc printed on one side and nickel-zinc printed on the other. One of these square batteries, which measures 4 centimeters wide, could power a small electric fan for at least 45 minutes. They also reported that bending or twisting the battery did not interrupt the power supply. Another experiment showed that even when they cut away parts of the battery, the LED connected to the device remained lit, suggesting that it can still perform even under normal wear and tear.
When it comes to this new work, one potentially interesting question is whether "this battery can in the future utilize sweat as the activation electrolyte and realize self-powered wearables," Fan, who was not involved in this new study, tells Inverse. "Such a design will also enable health monitoring."







