Roughly 20 years ago, a biologist named Caroline Gargett went in search of some remarkable cells in tissue that had been removed during hysterectomy surgeries. The cells came from the endometrium, which lines the inside of the uterus. When Dr. Gargett cultured the cells in a petri dish, they looked like round clumps surrounded by a clear, pink medium. But examining them with a microscope, she saw what she was looking for — two kinds of cells, one flat and roundish, the other elongated and tapered, with whisker-like protrusions.
Dr. Gargett strongly suspected that the cells were adult stem cells — rare, self-renewing cells, some of which can give rise to many different types of tissues. She and other researchers had long hypothesised that the endometrium contained stem cells, given its remarkable capacity to regrow itself each month. The tissue, which provides a site for an embryo to implant during pregnancy and is shed during menstruation, undergoes roughly 400 rounds of shedding and regrowth before a woman reaches menopause. But although scientists had isolated adult stem cells from many other regenerating tissues — including bone marrow, the heart, and muscle — “no one had identified adult stem cells in endometrium,” Dr. Gargett says.
Several types of self-renewing cells
Such cells are highly valued for their potential to repair damaged tissue and treat diseases such as cancer and heart failure. But they exist in low numbers throughout the body, and can be tricky to obtain, requiring surgical biopsy, or extracting bone marrow with a needle. The prospect of a previously untapped source of adult stem cells was thrilling on its own, says Dr. Gargett. And it also raised the exciting possibility of a new approach to long-neglected women’s health conditions such as endometriosis.
Before she could claim that the cells were truly stem cells, Dr. Gargett and her team at Monash University in Australia had to put them through a series of rigorous tests. First, they measured the cells’ ability to proliferate and self-renew, and found that some of them could divide into about 100 cells within a week. They also showed that the cells could indeed differentiate into endometrial tissue, and identified certain tell-tale proteins that are present in other types of stem cells.
Dr. Gargett, who is now also with Australia’s Hudson Institute of Medical Research, and her colleagues went on to characterise several types of self-renewing cells in the endometrium. But only the whiskered cells, called endometrial stromal mesenchymal stem cells, were truly “multipotent,” with the ability to be coaxed into becoming fat cells, bone cells, or even the smooth muscle cells found in organs such as the heart.
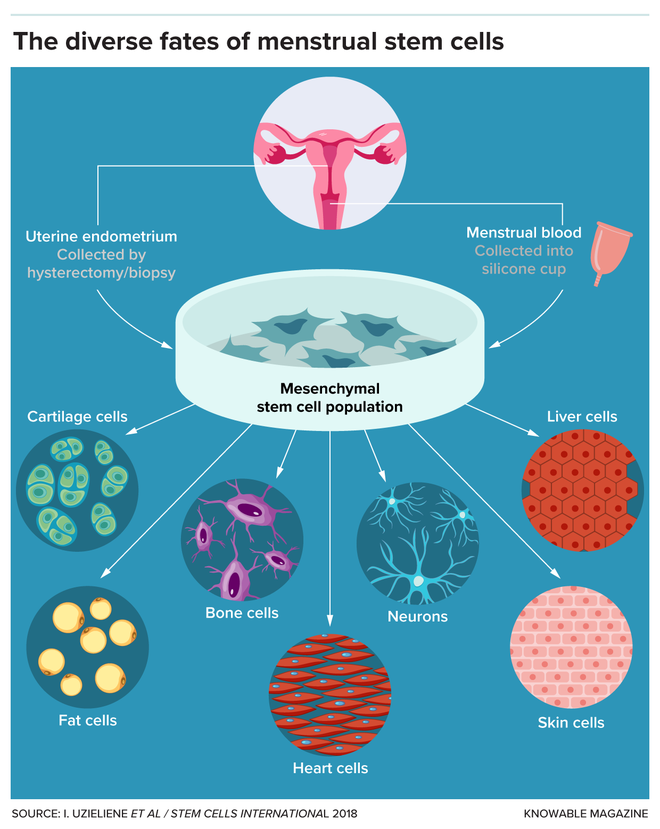
Special stem cells
Around the same time, two independent research teams made another surprising discovery: Some endometrial stromal mesenchymal stem cells could be found in menstrual blood. Dr. Gargett was surprised that the body would so readily shed its precious stem cells. Since they are so important for the survival and function of organs, she didn’t think the body would “waste” them by shedding them. But she immediately recognised the finding’s significance: Rather than relying on an invasive surgical biopsy to obtain the elusive stem cells she’d identified in the endometrium, she could collect them via menstrual cup.
More detailed studies of the endometrium have since helped to explain how a subset of these precious endometrial stem cells — dubbed menstrual stem cells — end up in menstrual blood. The endometrium has a deeper basal layer that remains intact, and an upper functional layer that sloughs off during menstruation. During a single menstrual cycle, the endometrium thickens as it prepares to nourish a fertilised egg, then shrinks as the upper layer sloughs away.
Dr. Gargett’s team has shown that these special stem cells are present in both the lower and upper layers of the endometrium. The cells are typically wrapped around blood vessels in a crescent shape, where they are thought to help stimulate vessel formation and play a vital role in repairing and regenerating the upper layer of tissue that gets shed each month during menstruation. This layer is crucial to pregnancy, providing support and nourishment for a developing embryo. The layer, and the endometrial stem cells that prod its growth, also appears to play an important role in infertility: An embryo can’t implant if the layer doesn’t thicken enough.
Testing for endometriosis
Endometrial stem cells have also been linked to endometriosis, a painful condition that affects roughly 190 million women and girls worldwide. Although much about the condition isn’t fully understood, researchers hypothesise that one contributor is the backflow of menstrual blood into a woman’s fallopian tubes, the ducts that carry the egg from the ovaries into the uterus. This backward flow takes the blood into the pelvic cavity, a funnel-shaped space between the bones of the pelvis.
Endometrial stem cells that get deposited in these areas may cause endometrial-like tissue to grow outside of the uterus, leading to lesions that can cause excruciating pain, scarring and, in many cases, infertility.
Researchers are still developing a reliable, non-invasive test to diagnose endometriosis, and patients wait an average of nearly seven years before receiving a diagnosis. But studies have shown that stem cells collected from the menstrual blood of women with endometriosis have different shapes and patterns of gene expression than cells from healthy women.
Several labs are working on ways to use these differences in menstrual stem cells to identify women at higher risk of the condition, which could lead to faster diagnosis and treatment. Menstrual stem cells may also have therapeutic applications. Some researchers working on mice, for example, have found that injecting menstrual stem cells into the rodents’ blood can repair the damaged endometrium and improve fertility.
Beyond gynaecological diseases
Other research in lab animals suggests that menstrual stem cells could have therapeutic potential beyond gynaecological diseases. In a couple of studies, for example, injecting menstrual stem cells into diabetic mice stimulated regeneration of insulin-producing cells and improved blood sugar levels. In another, treating injuries with stem cells or their secretions helped heal wounds in mice.
A handful of small but promising clinical trials have found that menstrual stem cells can be transplanted into humans without adverse side effects. Dr. Gargett’s team is also attempting to develop human therapies. She and her colleagues are using endometrial stem cells — those taken directly from endometrial tissue, rather than menstrual blood — to engineer a mesh to treat pelvic organ prolapse, a common, painful condition in which the bladder, rectum or uterus slips into the vagina due to weak or injured muscles.
The condition is often caused by childbirth. Existing treatments use synthetic meshes to reinforce and support weak pelvic tissues. But adverse immune reactions to these materials have led these meshes to be withdrawn from the market. Dr. Gargett’s research — so far conducted only in animal models — suggests that using a patient’s own endometrial stem cells to coat biodegradable meshes could yield better results.
Misogyny and cultural taboos
Despite the relative convenience of collecting adult multipotent stem cells from menstrual blood, research exploring and utilising the stem cells’ power — and their potential role in disease — still represents a tiny fraction of stem cell research, says Daniela Tonelli Manica, an anthropologist at Brazil’s State University of Campinas. As of 2020, she found, menstrual stem cell research accounted for only 0.25% of all mesenchymal cell research, while bone marrow stem cells represented 47.7%.
Dr. Manica attributes the slow adoption of menstrual stem cells in part to misogynistic ideas that uteruses are outside the norm, and to reactions of disgust. “There’s certainly something of an ‘ick factor’ associated with menstrual blood,” agrees Victoria Male, a reproductive immunologist at Imperial College London who coauthored an article about uterine immune cells in the 2023 Annual Review of Immunology.
Cultural taboos surrounding menstruation — and a general lack of investment in women’s health research — can make it difficult to get funding, says Dr. Gargett. Immunologist Dr. Male has faced similar challenges — it was easier to obtain funding when she used to study immune cells in liver transplantation than it is now that she works on immune cells in the uterus, she says.
“If we want more research on menstrual fluid, we need more funding,” says Dr. Male, noting that the logistics of collecting menstrual fluid over multiple days can be expensive. For that to happen, “we have to tackle sex and gender bias in research funding.” Through more equitable investments, she and others hope, menstruation will be recognised as an exciting new frontier in regenerative medicine — not just a monthly inconvenience.
Sneha Khedkar is a biologist-turned freelance science journalist based out of Bengaluru. This article is republished from Knowable Magazine.







