
Mercury pollution is a global threat to human health, especially to unborn babies and young children. Exposure to methylmercury, a type that forms when mercury washes into lakes and streams, can harm children’s brain development and cause symptoms including speech impairment and muscle weakness in adults who consume seafood as their main food source. Methylmercury also threatens health and reproduction in fish and other wildlife.
Humans, animals and birds are exposed to methylmercury when they eat fish and shellfish. Scientists have been working for decades to understand how and when fish accumulate mercury. This information is key for assessing mercury risks across different water bodies and landscapes, and for evaluating policy changes designed to reduce mercury emissions.
For decades, scientists have used fish ear stones, known as otoliths, to gain insights into fish growth, migration, diet and the timing of their exposure to certain pollutants. These tiny structures of calcium carbonate, roughly the size of a pea, form inside fishes’ inner ears, where they help regulate hearing and balance. Otoliths can also provide clues about how climate change is affecting fish.
But some pollutants, including mercury, are not incorporated into otoliths. Rather, they bind very strongly to tissues that contain sulfur, such as muscle tissues. That’s why muscle tissues have historically been used to assess contamination due to mercury pollution.

In a newly published study, we describe a new window into individual fish’s lifetime exposure to mercury by measuring it in the fish’s eyes. This work is unlocking new possibilities for understanding fish lifetime exposure to this potent neurotoxicant.
Clues in fish ears and eyes
Today, scientists analyze mercury uptake in fish by measuring how much of it accumulates in whole bodies of fish, or often just in fillets – that is, muscle tissues. This approach tells us how much mercury the fish has accumulated over its lifetime, but it doesn’t tell us specifically when in its life the fish was exposed. A time stamp is missing.
Mercury concentrations can vary widely within any given fish species. For example, from 1991 to 2010, U.S. government monitors detected mercury levels in cod that averaged 0.111 parts per million but ranged as high as 0.989 parts per million, a ninefold difference. This suggests that in addition to changes in mercury emissions over time, an individual fish’s movements and diets can significantly affect its exposure.
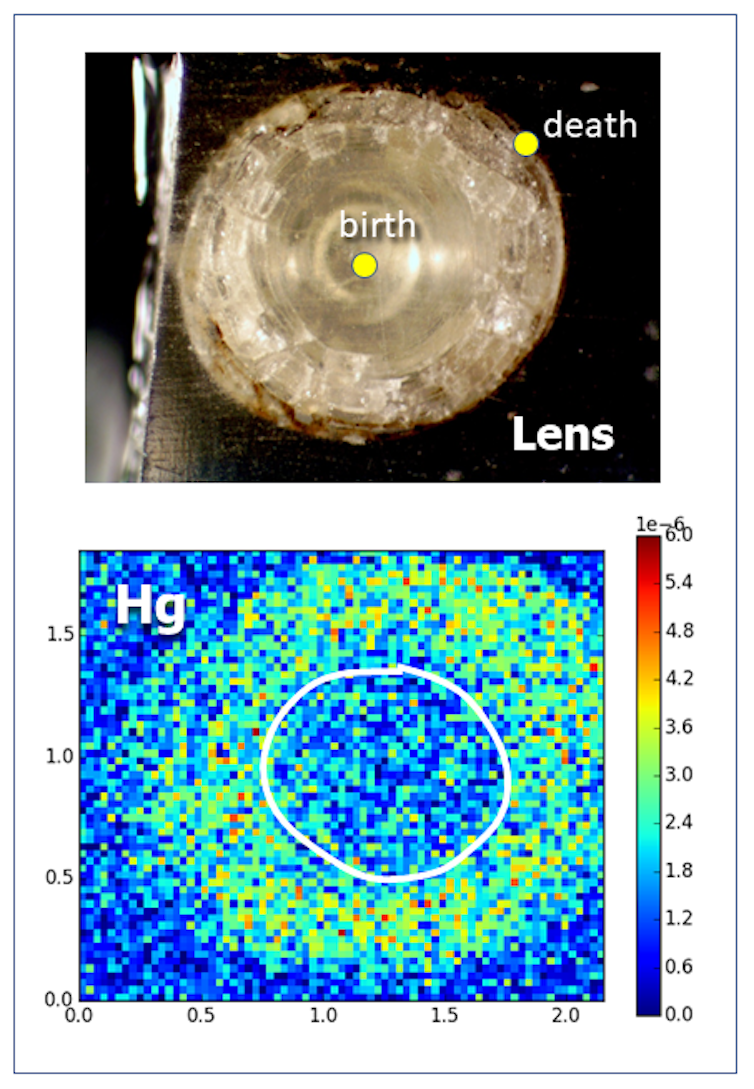
In our study, we propose a new method that combines measurements of otolith aging and of mercury in the lenses of a fish’s eyes to assign ages to fish eye mercury concentrations. Eye lenses are made of pure protein, are high in sulfur content and thus readily take up mercury either directly from water or from the fish’s diet.
Methylmercury appears to be preferentially taken up in certain organs, including eye lenses. At high doses, it may impair fish vision.
Our approach starts with the well-established technique of aging a fish using its otolith. As a fish grows and ages, its otoliths add yearly layers of calcium carbonate. We can estimate the fish’s age and growth rates by measuring the distance between the yearly growth layers, which are called annuli, much as foresters date trees by measuring the growth rings in their trunks.
We also know that a fish’s eye grows at a rate that is proportional to the growth of its otolith. So in our analysis, we apply the proportional distance that we found in the fish’s otolith to its eye lens. For our focal species, the Round Goby (Neogobius melanostomus), the linear relationship between these two measurements is strong.
As the eye lens grows and accumulates mercury, we can pinpoint when the fish was exposed using this correspondence with the otolith. And because the fish’s eye lens grows in layers throughout life, we can follow the chronology of lifetime exposure.
A possible climate connection
With this new method, we can start to trace the chronology of a fish’s lifetime mercury exposure. And we can ask questions about how life history events, such as migration and diet shifts, or temporal events such as low dissolved oxygen levels in water at certain times of year, may influence a fish’s mercury levels.
The strength of this method is that it provides information for individual fish, which matters just as it does for humans. Different individual fish have varying abilities to catch prey and avoid or tolerate stress, all of which can affect their growth and exposure to mercury.
And having information about mercury exposure for all ages of a single fish can help decrease the need to collect large samples of many fish across all ages, which is how scientists traditionally have assessed how fishes’ exposure changes over their lifetimes.
This new method may also help us understand how climate change is affecting mercury exposure.
As water temperatures rise, rivers, lakes, estuaries and oceans are losing some of their dissolved oxygen. This process, known as deoxygenation, is a critical stressor for aquatic life.
When oxygen in a pond or bay falls below 2 milligrams per liter, compared with normal levels of 5 to 8 milligrams per liter, that water body is said to be hypoxic – and hypoxic conditions can be associated with elevated concentrations of methylmercury. This loss of oxygen is exacerbated by nutrient pollution – for example, from urban or agricultural runoff. But it can also occur in the open oceans, far from continents, due to warming.
Increasing hypoxia could negate recent global efforts to reduce mercury emissions by making the mercury that is already in lakes and oceans more available for uptake into fish. However, fish response to hypoxia can vary by individual and by species. Our current research, sponsored by the National Science Foundation, is exploring how fish eye lenses, together with otoliths, can help us disentangle exposure to mercury from diet and hypoxia.
Increasingly, scientists are recognizing that various body parts of organisms function as archives of the past. For us, eye lenses and otoliths serve as key means to understand the secret lives of individual fish.
Roxanne Razavi receives funding from the U.S. National Science Foundation.
Hadis Miraly is supported by funding from the U.S. National Science Foundation.
Karin Limburg receives funding from the U.S. National Science Foundation, which sponsored this research.
This article was originally published on The Conversation. Read the original article.







