
Scientists have devised a method to hijack the human eye, enabling it to see brand-new colors that lie beyond the scope of natural human vision.
With this technique, the researchers enabled five people to see a new color, dubbed "olo," which the study participants described as a "blue-green of unprecedented saturation." The researchers, some of whom participated in the experiment themselves, described their technique and the new color in a study published Friday (April 18) in the journal Science Advances.
"The ultimate goal is to provide programmable control over every photoreceptor [light-sensing cell] in the retina," primarily for research purposes, said co-first author James Fong, a doctoral student in computer science at the University of California, Berkeley. "Although this has not been achieved to that level, the method we present in the current study demonstrates that a lot of the key principles are possible in practice," Fong told Live Science in an email.
Controlling the retina at this granular level could open up new ways of studying vision, the researchers said. For instance, scientists could use the system to replicate the effects of different eye diseases to better understand the vision loss they trigger. In theory, the technique could also be used to simulate full-color vision in people who are color-blind, essentially compensating for their missing or defective photoreceptors.
By using the system to introduce the brain to new visual data and patterns of retina stimulation, in theory, "it may be possible that this [color-blind] person would learn to see the new dimension of color," Fong suggested.
Related: This optical illusion tricks you into seeing different colors. How does it work?
Journey to Oz
Human eyes contain light-sensitive cells, called photoreceptors, which come in two forms: rods and cones. Rods enable night vision, as they respond to relatively low levels of photons, or packets of electromagnetic radiation.
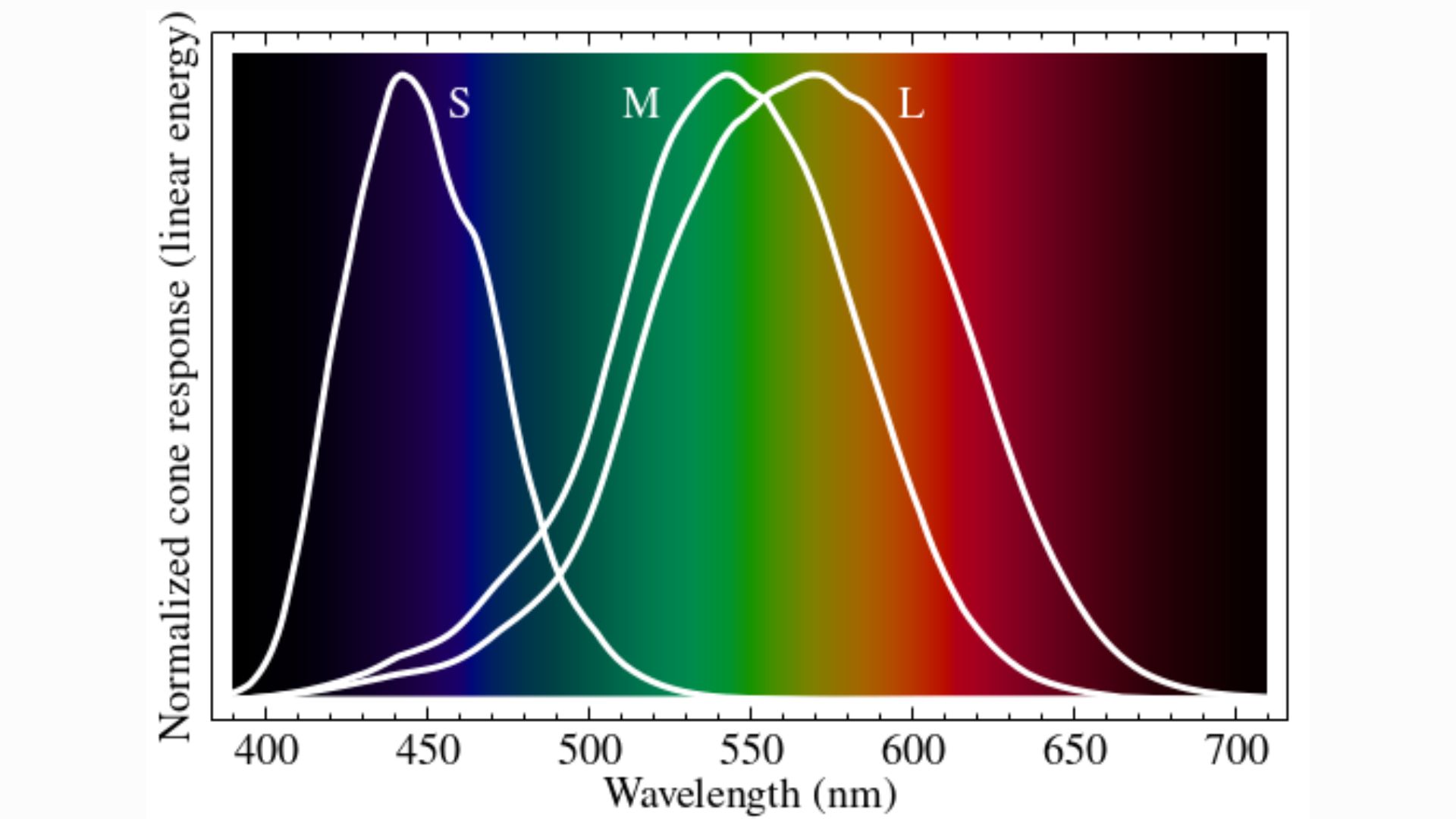
Cones take over in bright light, and they are specialized to detect specific wavelengths of visible light — namely, red, green and blue. These three types of cones are respectively named "L," "M" and "S," in reference to the long, medium and short wavelengths of the visible spectrum to which they are most sensitive.
Once cones are activated, color vision relies on the brain to interpret the activation patterns of these three types of cells across the retina. Each pattern acts like a code, with different codes unlocking different perceptions of colors and intensities of light.
M cones are most sensitive to green, but technically, they respond to a whole spectrum of colors that completely overlaps with the wavelengths L and S cones react to. As such, in natural conditions, you can't activate M cones without also activating L and S cones. The scientists wondered what would happen if you could defy that rule and exclusively activate M cones.
"We originally started this project specifically to study M cone stimulation," Fong said. "But it quickly became clear to us that [the] required underlying technology would be broadly useful to study visual function at a new level of scale and precision."
They named their resulting retinal stimulation technique "Oz," in homage to the green-tinted glasses that people in the Emerald City wear in the original "Wizard of Oz" books. The approach requires a detailed map of each user's retina. To create such a map, the researchers started by taking multiple videos of the retina and stitching them together to capture what the tissue looked like.
From there, the L, M and S cones were labeled; the locations of these cells are unique in each person's retina, Fong noted. To reveal each cone's identity, the researchers used a technique called adaptive optics optical coherence tomography (AO-OCT), which involved shining light on the cells and measuring how they changed shape; this response differs depending on which wavelengths a cone is sensitive to.
With a detailed retinal map, the team then ran their experiments. Each participant sat in front of a display with a small square at its center, where the Oz stimulation unfolded. The stimulation targeted specific types of cones with visible-wavelength laser light, called laser microdoses. So, to switch on only M cones, the system targeted only those cells with lasers.
The scientists also used a real-time feed of the eye during the experiment, and the approach accounted for the eye's subtle motion, to ensure the lasers hit their targets.
Revealing a new color
Stimulating only M cones revealed the color olo, whose name refers to coordinates on a 3D map of color — "0, 1, 0." The "o" is a zero, referencing the lack of stimulation of L and S cones, while the "l" is a 1, indicating full stimulation of M cones. After stimulating olo in isolation, the scientists were also able to incorporate the color into images and videos viewed by the participants.
One way to imagine olo is to think of the light from a green laser pointer and then turn up the saturation. In comparison with olo, monochromatic laser light looks "pale," some of the participants said. "It is very foreign to me to imagine how something else could be saturated enough to where the laser starts looking pale in comparison," Fong said.
Although Oz can already push the boundaries of human vision, it does have some limitations in its current setup.
For instance, participants cannot look directly at the Oz display, Fong noted, because the cones at the very center of the retina are very small, making it difficult to localize the laser light. Because of this, people in the study viewed Oz with their peripheral vision by looking at a fixed point slightly away from the square.
Eventually, Oz could potentially be applied on the fovea — the central part of the retina that enables super-sharp vision — but "it will be a significant challenge in practice," Fong said.
Another limitation is that, currently, users must fix their gaze in one spot to use Oz, because the scientists mapped only a small portion of the retina containing thousands of cones, as a proof of concept. Allowing people to shift their gaze freely would introduce "substantial technical challenges," the authors wrote in their paper. That's because more of the retina would need to be mapped and the method for delivering microdoses would need to be extraordinarily precise in tracking eye movement.
The scientists are now exploring the idea of using Oz to study and treat color blindness, as well as to stimulate the experience of having a fourth type of cone cell. This occurs naturally in some people and results in a rare ability called tetrachromacy, which boosts their sensitivity to color. The team is also using Oz to model various eye diseases.
Outside of scientific research, Oz could theoretically be used for everyday color displays, like those in your television or phone screen — but that application seems very unlikely, Fong said.
"Our current method depends on highly specialized lasers and optics that are definitely not coming to smartphones or TVs any time soon," he said. So, for now, olo will remain a rare color seen by only a few.







