The mangroves of the Caribbean bristle with life. White herons spear fish with their bills and black and red crabs skitter over the sprawling, spidery tree roots.
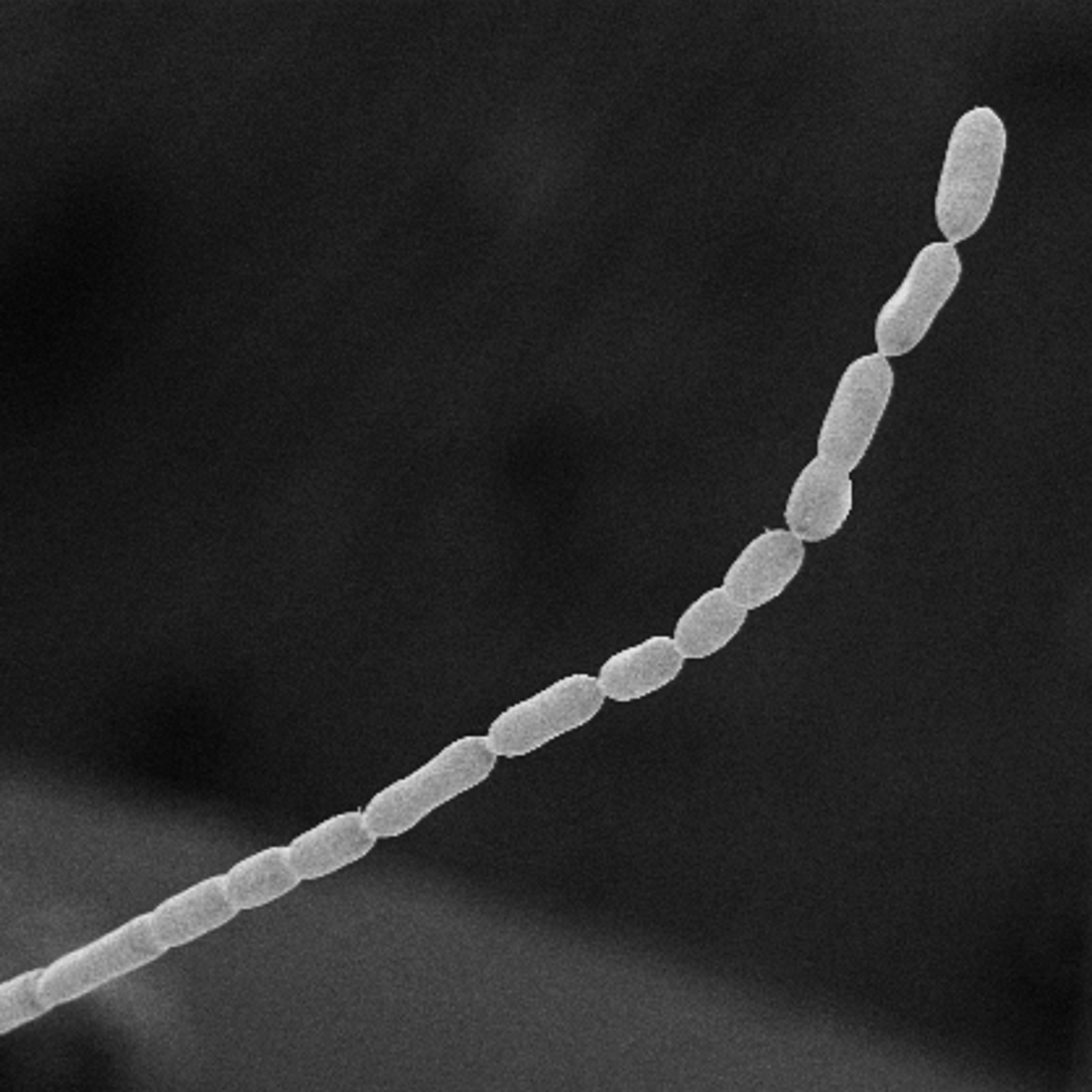
And then there are the thin, white filaments floating in the brackish water. They may not look like much, but comparatively, these little organisms are gigantic — both in size and scientific importance. This is Ca. Thiomargarita magnifica, the largest bacteria known to science, and it’s breaking all our rules.

What’s new — In a study published Thursday, June 23 in Science, researchers unveiled their discovery of these massive bacteria lurking in the mangroves of Guadeloupe. T. magnifica is not the first giant bacteria, but it sure is the largest, visible without a microscope and roughly twelve times longer than the bacteria that previously held the record, another member of the Thiomargarita family.
“They are the size and the shape of an eyelash,” says Jean-Marie Volland, a co-author of the study who works at the Department of Energy Joint Genome Institute, as well as the Laboratory for Research in Complex Systems, both in California.
That’s 4,500 times longer than most bacteria, which are invisible to the naked eye.
“This sets a new record that will be hard to beat,” says Danny Ionescu, who studies microbial ecology at the Leibniz Institute of Freshwater Ecology and Inland Fisheries in Berlin and was not involved in the new research.
Digging into the details — Researchers were in for yet another surprise when they looked at the inner workings of the giant bacteria. They used dye and molecular tools to illuminate its DNA, membranes, and ribosomes, which are the tiny structures that turn genetic code into proteins. They expected that fluorescence to be spread out across the cell. After all, bacteria are simple, and their DNA typically floats freely inside them. Only more complex types of organisms, such as members of the animal kingdom, keep their DNA bound inside designated cell structures.
But when the scientists looked under the microscope, they found the DNA and ribosomes clustered together in many tiny pockets, each surrounded by a membrane. No free-wheeling genetic information here.
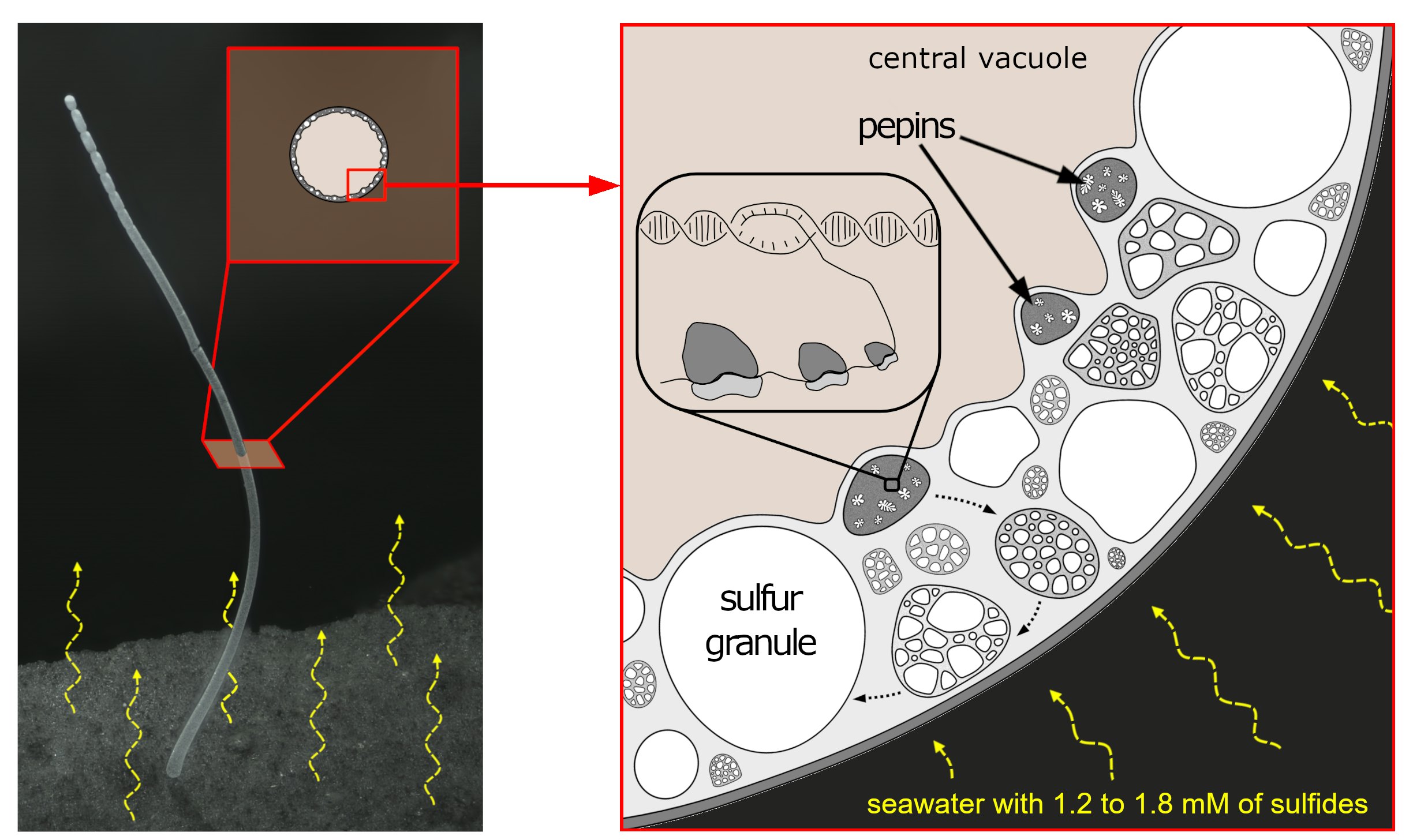
“That is not something we expected to see,” Volland says, so it took them time to understand what they were looking at. After further tests to eliminate other possibilities, the researchers were left with the conclusion that these were membrane-bound cell structures that organize about half a million copies of the bacteria’s genome. The researchers named these structures “pepins”, which means “seeds” in French, because they resemble the seeds of fruits like watermelon or kiwi.
This “challenges established concepts in bacterial genetics, indeed,” says Fabiano Thompson, who studies marine microbiology at the Federal University of Rio de Janeiro.
Embracing complexity — There’s a reason we expect simplicity from bacteria — because we have defined bacteria as simple organisms. Bacteria are a type of prokaryote, or a single-celled organism that has little internal structure. Contrast this with eukaryotes, the branch of life’s family tree to which the rest of us belong. These more complex, often multicellular organisms diverged from prokaryotes some 2.7 billion years ago, and have numerous tiny structures called organelles within each cell.
Obviously, T. magnifica is not your average prokaryote. “The cell structure of these giant bacterial cells differs significantly from our concept of a ‘normal’ bacterial cell,” says Sina Schorn, who studies other large bacteria at the Max Planck Institute for Marine Microbiology, who was not involved in the new study. “In my opinion, such compartmentalization of DNA might even be reminiscent of more complex life forms, such as unicellular eukaryotes,” Schorn says.
This is far from the first time that scientists have run head-first into their own assumptions. In the early 2000s, scientists struggled to identify a strange microorganism. It couldn’t be a virus — it was the size of a bacteria. Since viruses were discovered in 1892, they had been defined by their minuscule size, so small that they couldn’t be seen under a regular microscope. But this mystery organism was indeed a giant virus, and it shook the field of virology.
“Now, we start to look everywhere and find these giant viruses everywhere,” Volland says. The researchers see giant viruses as an apt comparison to their findings, and a similar example of confirmation bias in our quest to label and organize the complexity of life.
“Biology has taken on a more dogmatic approach to many, many issues — this is how prokaryotes are, this is how eukaryotes are,” says Shailesh Date, co-author of the study and CEO of the Laboratory for Research in Complex Systems. “We need to step back from that, just because we know that we haven’t really explored the microbial world at all. So who knows what kinds of surprises there may be in there,” he says.
What’s next? — The discovery of Thiomargarita magnifica has raised a seemingly infinite supply of questions — “Too many to describe,” says Date. “Every person on the team has an idea as to what to do next.”
But the first step in this quest is figuring out how to keep T. magnifica alive and reproducing in a laboratory, the authors say. That task is much more challenging than it sounds. The bacteria’s natural home, Guadeloupe’s mangroves, are complex ecosystems, and T. magnifica lives at the intersection of two very different worlds: the oxygen-rich seawater and sulfide-rich sediment. The bacteria rely on elements of both to survive, but these two ingredients just aren’t stable enough in the laboratory.

“Recreating those environments is pretty difficult,” says Tanja Woyke, co-author of the study and a microbiologist at the Joint Genome Institute. “It’s possible, but it’s challenging.”
If the researchers are able to cultivate T. magnifica in the lab, it will open up a whole new world of possibilities. High on the priority list is sequencing its whole genome — right now, the researchers only have reassembled fragments of its genetic code.
The researchers also hope to figure out how these pepin structures develop and what they have to do with its size. Because the bacteria are so big, it’s possible that having these pockets of DNA and ribosomes allows each region of the cell to respond better to its environment, the authors suggest. This ability could be especially important in the mangrove environment, where gradients of nutrients like oxygen and sulfur may provide a different environment to each end of its long cell.
“We try to understand the boundaries of life and the complexity of life,” Volland says of his research group — and there’s no question that the magnificent Thiomargarita magnifica lives at the boundaries of what we think we know.