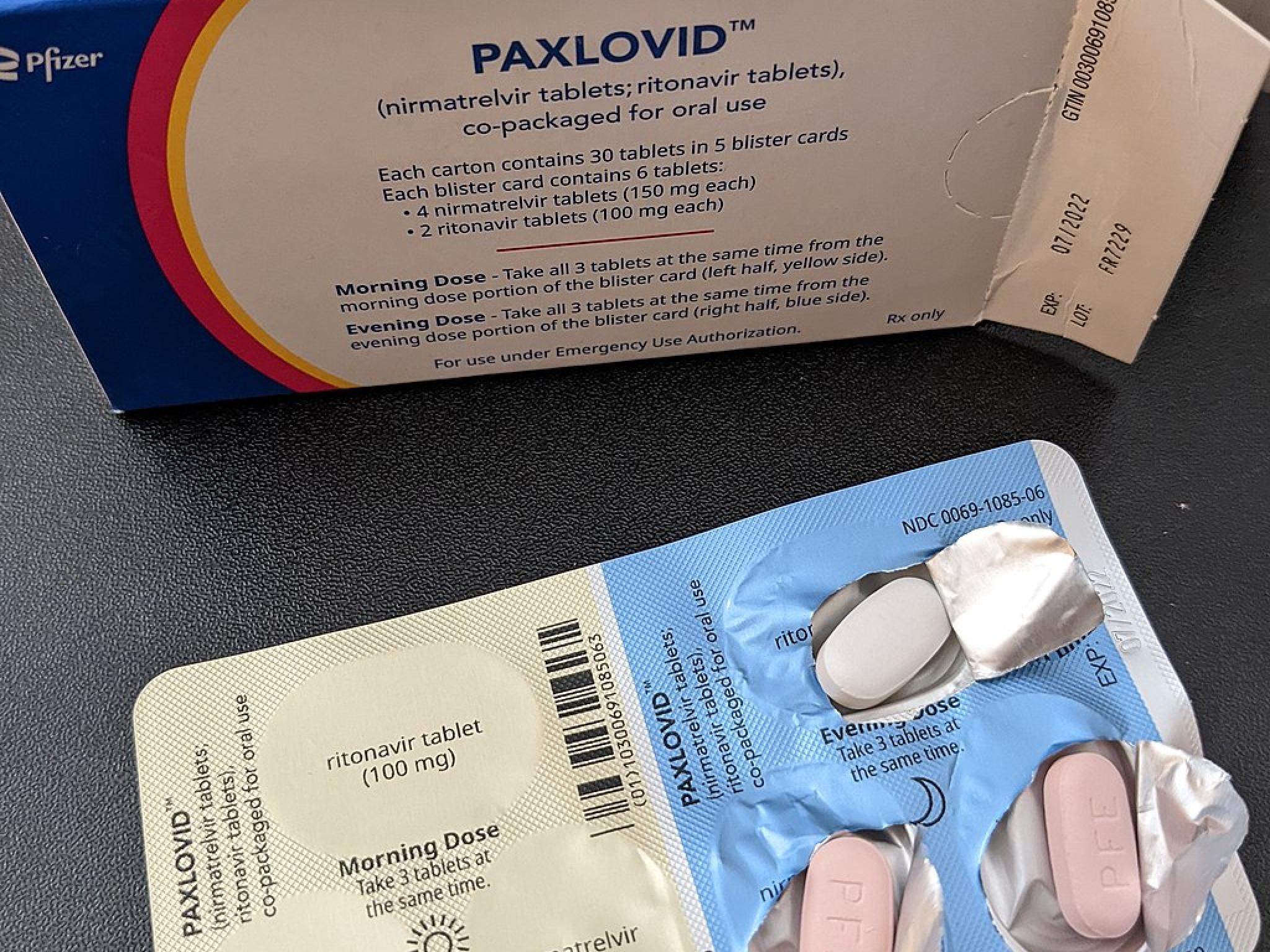
- The U.S. National Institutes of Health and Pfizer Inc (NYSE:PFE) are discussing testing a longer course of COVID-19 antiviral treatment Paxlovid, required to prevent reinfections, White House Chief Medical Officer Anthony Fauci said.
- "We're going to be planning what studies we're going to be doing relatively soon, within the next few days," to determine whether a longer course is needed, Fauci said during a White House COVID-19 briefing.
- White house estimates that about 20,000 prescriptions of Paxlovid are being given out every day.
- Also Read: COVID-19 Symptoms Rebound After Pfizer's COVID-19 Therapy, What Could Be The Reason?
- Pfizer has suggested that a second five-day course of Paxlovid could treat reinfections. The FDA said there is currently no evidence to support taking a second five-day course or a 10-day course of the pills.
- In Pfizer's clinical trial, around 2% of recipients who received the two-drug treatment saw an increase in viral load after completing the standard course, compared with around 1.5% of placebo recipients.
- Price Action: PFE shares are up 0.07% at $50.44 during the market session on the last check Thursday.