
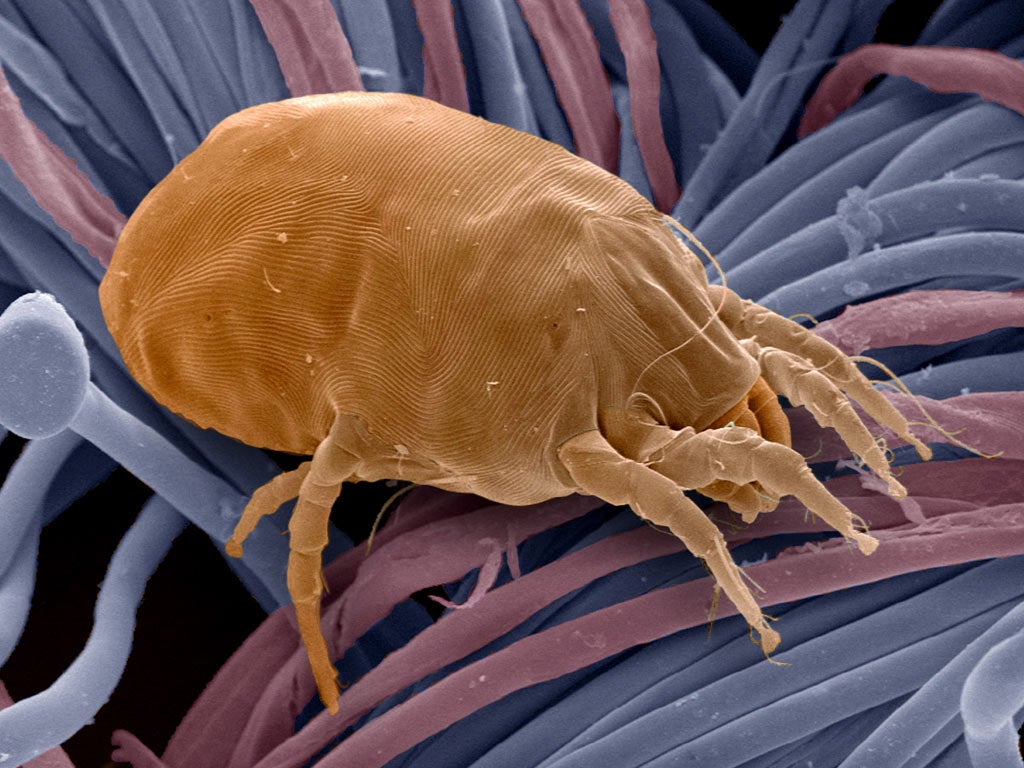
A new, first-of-its-kind tablet offering relief from severe dust mite allergies has been approved for NHS use, potentially benefiting thousands.
The National Institute for Health and Care Excellence (Nice) has given the green light to 12 SQ-HDM SLIT, also known as Acarizax, for individuals aged 12 to 65 suffering from persistent moderate to severe house dust mite allergic rhinitis.
This marks the first treatment Nice has recommended for this condition, estimated to affect around 13,000 people in England.
The tablet works by gradually desensitising the body to house dust mites. By administering a fixed daily dose of house dust mite allergy extract, the body builds resistance, reducing the severity of allergic reactions over time.
This is particularly beneficial for those whose symptoms don't adequately respond to traditional treatments like steroid nasal sprays and antihistamines.
The treatment is taken for three years at home to build up the body’s resistance.
Common symptoms of allergic rhinitis include sneezing, an itchy nose, a runny or blocked nose and itchy, red and watery eyes.
In severe cases it can be debilitating and cause facial swelling, fatigue and affect people’s sleep, Nice said.
Persistent allergy is when symptoms occur on four or more days a week for a month and have not been helped by standard treatments.
People will be eligible for the new pill after medics have looked at their medical history and if they test positive for house dust mite allergy through the allergy skin prick test or specific immunoglobulin E [IgE]) test.
Helen Knight, director of medicines evaluation at Nice, said: “For people with house dust mite allergic rhinitis that is not controlled by standard treatments, this new cost-effective drug will have a significant positive impact on their quality of life.
“This is a chronic, debilitating condition which can prevent people from going to their workplace or school.
“This medicine has been found to improve symptoms, helping people to live their lives and has potential to be truly life changing.”
Dr Helen Evans-Howells, a patient expert and a GP, said: “This drug could be life-changing for those affected by severe symptoms which include significant fatigue, congestion and facial swelling.
“The evidence clearly shows it offers an effective solution for those who have struggled with standard treatments.”
Amena Warner, head of clinical services at Allergy UK, said the decision was a “landmark step”.
“Many endure years of misery; of nasal congestion, loss of smell and sneezing, impacting their sleep and daily living with little respite from symptoms.
“This can also affect mental wellbeing. With this treatment there is now hope for people who fit the criteria to be able to access this through the NHS.”







