
Mars didn’t have an oxygen-rich atmosphere 3.5 billion years ago after all, according to a recent lab experiment.
Minerals found on Mars in 2014 looked like proof that Mars once had an atmosphere teeming with oxygen, because those same minerals formed in wet, oxygen-rich environments here on Earth. But in recent experiments, Stony Brook University planetary scientist Kaushik Mitra and his colleagues found that it doesn’t take oxygen to kickstart the chemical reactions that produce those minerals after all.
In fact, oxygen isn’t actually very good at starting the necessary chemical reactions. That may mean that ancient Mars didn’t have an atmosphere rich in oxygen, which could have some interesting implications for the search for evidence of past life on Mars.
Mitra and his colleagues published their results in the journal Nature Geoscience.
What’s new — In 2014, NASA’s Opportunity and Curiosity rovers found interesting minerals in some rocks from Endeavor and Gale craters. Those minerals, called manganese oxides, were various combinations of manganese (an element common in Earth’s crust) and oxygen atoms. Most manganese oxides here on Earth formed in a very wet environment, during a time in our planet’s past when the atmosphere contained even more oxygen than it does today. So the minerals’ presence on Mars looked like a smoking gun, pointed at an oxygen-rich atmosphere on ancient Mars.
But in recent lab experiments, Mitra and his colleagues found that chemicals like chlorate (a compound of chlorine and oxygen) or bromate (a compound of bromine and oxygen) could trigger the reactions that produce manganese oxides.
That was especially true in slightly acidic water, which Mitra and his colleagues mixed up in the lab to replicate what water on early Mars was probably like, according to geologists.
“Under many of these conditions, oxygen is altogether incapable of forming manganese oxides,” explains an announcement from the University of Washington in St. Louis.

Here’s The Background — For this to really make sense, we need to talk about chemistry.
Oxidation is a chemical reaction that takes away an electron from an atom or molecule. The first time chemists noticed this kind of reaction, it happened to involve oxygen, but lots of other chemicals can also cause oxidation reactions. Some of them are actually better at it than oxygen, especially under certain conditions.
Meanwhile, oxides are combinations of oxygen with some other chemical. The most familiar one, for most of us, is iron oxide, better known as rust. Oxides are just one example of what you can produce with an oxidizing reaction — and here’s an example of oxidizing reactions that don’t involve any oxygen anywhere, along with a chemical equation for making manganese oxide, in case chemical equations are your thing.
Clearly, you need some oxygen to make manganese oxides, since those oxygen atoms have to come from somewhere. But that oxygen can be mixed up with other chemicals, like in water, or in compounds like bromate or chlorate. You don’t need any oxygen at all to trigger the reaction that creates magnesium oxides (which is what Mitra and his colleagues just discovered), and you only need a little oxygen somewhere to actually make magnesium oxides.
In other words, you can get magnesium oxides even on a version of ancient Mars that doesn’t have a lot of oxygen floating around in its atmosphere. And that means that manganese oxide doesn’t actually prove Mars once had an oxygen-rich atmosphere. We have to rethink what we thought we knew.
Why It Matters — We know that most of Mars today is pretty inhospitable to life, and the burning question is whether anything lived on Mars in the past, between 3.5 and 4 billion years ago. And part of answering that question is figuring out what the environment on Mars was like at that time.
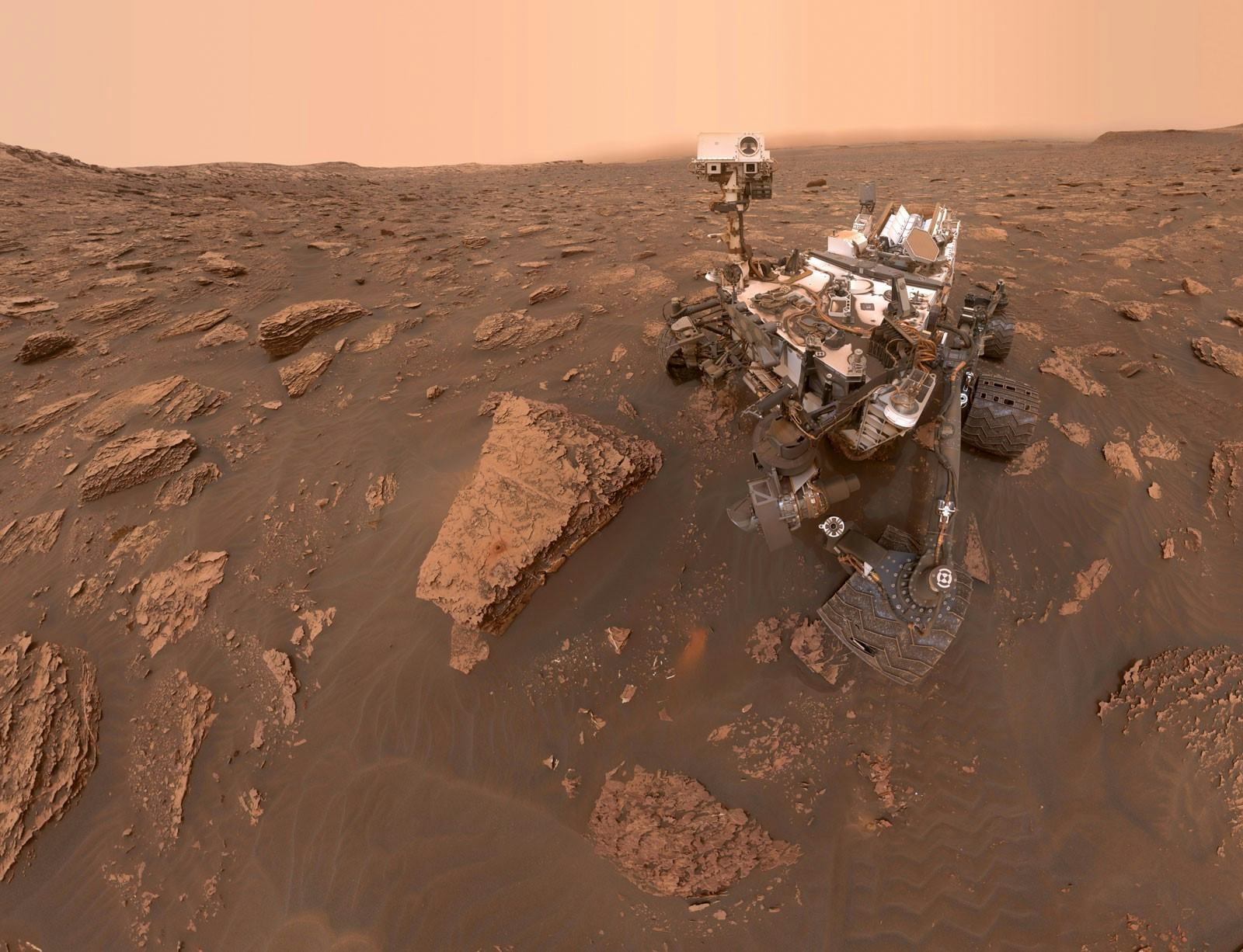
Rocks are our best clue about ancient environments, because every rock holds clues about the conditions that formed it. That’s part of why Curiosity has spent so much time zapping rocks with its lasers to find out what they’re made of, and why Perseverance is currently stockpiling interesting rocks to send back to Earth eventually. On Earth, most manganese oxide minerals formed under conditions with lots of water and an atmosphere laden with oxygen.
But there’s often more than one route to make a particular mineral (or any other chemical compound). That’s why, for example, the discovery of phosphine in the atmosphere of Venus stirred up such a debate. It’s important for scientists to consider all the possible explanations.
“Every planet is unique in its own right, and we cannot extrapolate the observations made on one planet to exactly understand a different planet,” says Mitra.
What’s Next — With that in mind, Mitra and his colleagues say experiments like theirs are an important way to explore how minerals could form with the ingredients available on very different planets, from icy moons like Europa to sulfuric hellscapes like Venus.
Meanwhile, the search for evidence of ancient life on Mars continues — and the lack of an oxygen-rich atmosphere only changes what kind of life scientists are looking for.
“There are several life forms even on Earth that do not require oxygen to survive. I don’t think of it as a ‘setback’ to habitability, only that there was probably no oxygen-based life forms,” says Mitra.







