
The sun is shining, and air feels surprisingly warm when we walk on a 35cm ice that covers a frozen lake in central Finland. The heavy sledges move nicely, because there is not much snow on the ice today. The journey isn’t far either, as we’re by the city of Kuopio, which is surrounded by Finland’s 10th largest lake. Despite the temperature of -10˚, I need to take off my hat – the sun in early March is already warm, or it could be the fact that the heavy sledge is following me obediently.
We’re crossing the icy bay not for sport or holiday activities, nor is it part of a plan to hike to north Pole. Instead, our focus is in research. We are determinedly walking to the middle of the bay to collect a sediment core from its bottom. Mud – or sediments, as geologists call them – are deposited slowly at the bottom of lakes. How quickly mud accumulates varies greatly depending on the body of water, but at this bay in Lake Kallavesi, about 1 centimetre of sediment is deposited annually. Logically, the new sediment is deposited on top of earlier layers, and so sediments are like time machines – the deeper you dig into the older sediments, the further you reach into the past. You can think of sediments like libraries of a lake’s untold stories, and if you can read the words of the sediment core, they can tell amazing stories.
Lake Kallavesi has a specific and rare type of sediments called annually laminated or varved sediments. They’re composed of a bright and dark couplets one after the other, just like tree rings, that can be counted backwards. It is possible to check how your birth year looked – or your grandmother’s birth year. Such sediment layers can reach back thousands of years.
The history of plastic, buried in the mud
Our historical destination this time is much more recent – we want to investigate the presence of plastic particles within the natural sediment. It’s a continuation of our ongoing research, most recently published in the Journal of Soils and Sediments in February 2023.
Widespread use of plastic started about 70 years ago, and since then, 9 billion metric tonnes has been produced. Only 12% is incinerated, meaning that 7.5 billion metric tonnes are still with us somewhere – recycled and in use; in landfills or dump pits, or in nature, including our waters. The weight of all that plastic is more than that of all the people on the planet – there’s about 1,000 kg of plastic for each of us, mostly in form of waste. What would you do with your share? What would I do?
These are my thoughts when I am drilling a hole in the ice. It would be nice to work on lake on a sunny summer day, but the thick ice serves as a stable platform. It allows us to spread all our corers, saws, sledges, tubes, wires, and hot water pots around us. We use metal rods to push the core tubes down 11 metres to the lake floor and then into the sediment. A few minutes later, we lift the core tube out on the water. It was known that the bay is polluted, but we’re surprised by the strong smell of oil when the core emerges.
Because plastic is very durable material, it works well as a core tube. This benefit is also plastic’s worst aspect: released into the environment, it doesn’t decompose but breaks into ever smaller pieces. Particles smaller than 5 mm are called microplastics, and they have only been studied since 2004, after Richard Thompson accidently noted their presence in coastal sediments near Plymouth, England. While it’s a relatively new research field, we already know that microplastics are harmful pollutants that endanger animal life – including our own – and that they are found everywhere from the top of the Himalayas to the deepest oceans.
Like natural particles, microplastics are transported to the lakes by rivers, rainfall, and wind. They can float in the surface but finally sink to the bottom. There they will be slowly buried under new layers of sediments. But how much microplastics has increased in the nature since the last 70 years? Let’s go to see what the sediment library can tell us.
The ABCs of reading sediment layers
The 2-meter sediment core lies on the metallic table at our laboratory. As we saw open the core, my skin gets goosebumps. It might be the noise or maybe it is just excitement – after all, you never know beforehand what the sediment will look like.

Sediments consist of natural materials as well as pollutants. Detrital materials such as clay, silt and sand are washed into the lake by spring floods that follow the melting of snow – this is the bright layer in Lake Kallavesi sediment. The thicker the bright layer is, the more intensive the spring flood and higher the snow was during the winter.
There is also a lot of organic matter in the sediments – not only plants transported by the rivers and pollen flown in from long distances, but also algae. On sunny summer days, they bloom on the lake’s surface and so serve as a buffet for the zooplankton that graze on the surface. When these microscopic organisms die, they too sink to bottom and become part of the mud.
Sediments also bear witness to human activities. Building a bridge or a road involves digging and can increase erosion, and our sediment shows bright layers that can be several centimetres thick. A significant number of pollutants are buried within the sediments – we found trace metals such as mercury, copper, lead and zinc as well as petroleum hydrocarbon fractions and PAH compounds that are ecologically risky and potentially dangerous to health. Many are related to burning of fossil fuels. In addition to this chemical cocktail, the sediments were flavoured by a large amounts of microplastics.
Occasionally I get the feeling that I never went too far from my childhood. Playing with water and mud was the greatest thing I could imagine for the summer holidays, and nowadays I keep on doing very similar activities – collect mud, treat it in different ways, put it in all kinds of cups and machines. I often come home with my clothes splashed with mud. Today, however, I’m planning my playing in more detail, having spent weeks in the laboratory preparing these sediments for analysis.
Two steps forward, one steps back
The preliminary results show that the amount of heavy metals and oil fractions have decreased significantly from the peak in the 1970s toward the present day. This is good news, because it tells us that we’ve come to understand the harmfulness of these chemicals and our actions to preserve nature have paid off. Unfortunately, that’s not the case for microplastics – their presence in the sediments is increasing over time.
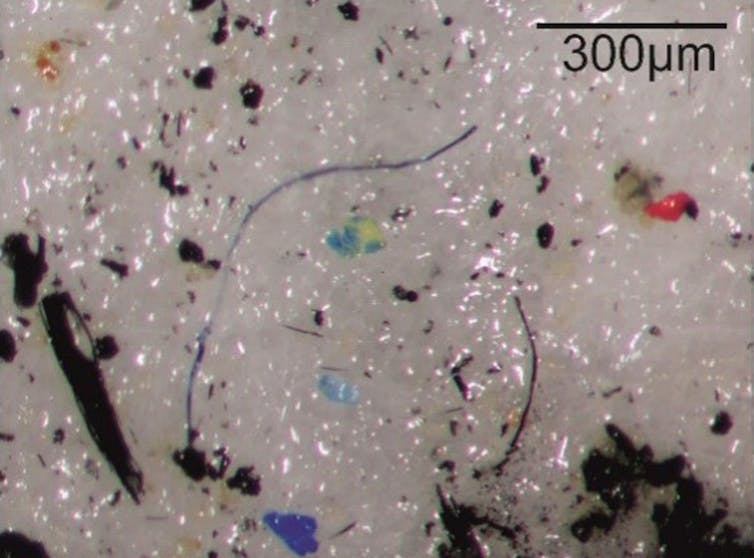
The materials most frequently found are polyethylene, polypropylene and polystyrene, often employed for so-called single-use products such as packaging. In the annual layers we can immediately find the years 2011-2013, when there was significant construction work and dredging in the harbour. During this period, a huge number of microplastics are present with a large diversity of types.
With such detailed information, we start to understand how human activities on the land have a direct influence on the microplastics in the water. In the future, we want to understand how all kinds of pollutants that are already in the nature can be attached to microplastic particles, and what when such particles are eaten by plankton and animals that graze on the bottom of lakes. There is still much we do not understand from microplastics and the risks they pose, but our knowledge increases with every sediment core. It is not piece of cake, but a mud cake.

Created in 2007 to help accelerate and share scientific knowledge on key societal issues, the Axa Research Fund has supported nearly 700 projects around the world conducted by researchers in 38 countries. To learn more, visit the site of the Axa Research Fund or follow on Twitter @AXAResearchFund.
Saija Saarni a reçu des financements de AXA Research Fund.
This article was originally published on The Conversation. Read the original article.







