
Lyme disease is the leading vector-borne disease — meaning diseases that are transmitted to humans from another organism like a tick or mosquito — in North America and Europe.
New human cases are estimated at over 400,000 in the United States each year. Canada has experienced a drastic increase in human cases, from 266 cases in 2011 to 3,147 in 2021, as the habitat of its vector, a tick, expands north.
The initial symptoms of human Lyme disease can be vague, such as fever, headache, fatigue and often rash. It is a potentially serious condition that can affect multiple systems in the body — including the heart, nervous system and joints — and can become a chronic illness.
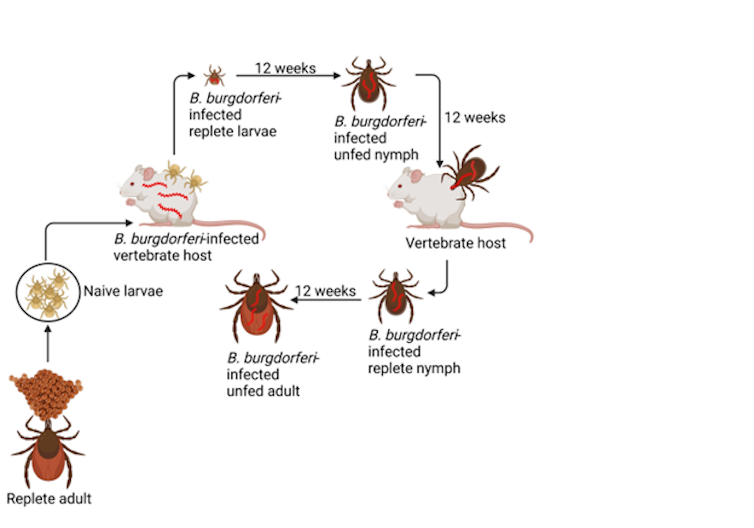
Lyme disease is caused by a unique, spiral-shaped (spirochete) bacterium called Borrelia burgdorferi. B. burgdorferi cannot survive in the environment on its own. For survival and transmission, it requires susceptible hosts (usually small mammals or birds) and a specific vector: the black-legged tick, also called the deer tick.
Evading the immune system
B. burgdorferi must survive extremely diverse conditions over the course of its transmission and infection cycle: from host to tick vector, and then into new hosts.
This bacterium senses and responds to its surroundings, most notably by modifying its appearance by changing the proteins on its outer surface to help it survive in either the tick or the host.
When a tick infected by B. burgdorferi bites and feeds on a vertebrate host, it provides a signal for the bacteria to switch its proteins to those required to infect the host, and to begin migrating through the tick and into the bite site. This process takes between 36 and 72 hours.
However, many of these proteins are recognized by the host as foreign, and the host’s immune system works to try to clear the infection. This includes a strong, antibody response targeted against B. burgdorferi.
Despite these immune responses, B. burgdorferi is able to cause long-term infections. In natural host reservoirs — the animals that the bacterium usually finds itself in via tick bites, such as small rodents — these infections do not cause diseases like those seen in humans and other non-natural reservoirs.
In fact, the bacteria itself does not produce any products that would be toxic to its hosts, either natural or non-natural. Yet chronic infection in humans can lead to Lyme neuroborreliosis, carditis and Lyme arthritis.
How then, are these bacteria able to cause such a devastating disease in humans and other animals, but not in their natural host reservoirs?
While there is still much to learn about B. burgdorferi, we know of several factors that play a role in the range of disease it causes. These include:
- its genetic make-up,
- its ability to access various tissues (such as the joints, heart and nervous system) due to its ability to move around (motility), and
- the immune response of the host.
Apart from motility, B. burgdorferi also protects itself from the strong B. burgdorferi-specific targeted antibody response of its host’s immune system by changing the appearance of the main outer surface protein expressed during persistent infection in a process called antigenic variation.
How Lyme disease is perpetuated
In addition to antigenic variation, B. burgdorferi bacteria can also change their DNA by exchanging genetic information, a process also known as gene transfer. This process allows these bacteria to further alter their appearance during infection to avoid the host immune system.
This process works so well that these B. burgdorferi bacteria appear different enough to allow re-infection or even co-infection (where multiple strains of B. burgdorferi infect a single host at the same time) of a vertebrate host, like a mouse or a human, despite the presence of specific antibodies to fight the bacterium.
In fact, in nature, the majority of host reservoirs and the ticks that carry the bacterium are infected with multiple strains of B. burgdorferi. The ability of B. burgdorferi to reinfect and co-infect both ticks and hosts increases the spread of the bacteria in the environment as well as the chances that humans will encounter Lyme disease.
Human cases of Lyme disease are increasing
As a vector-borne pathogen, B. burgdorferi only infects individuals that are bitten by an infected tick. It is not transmitted from person to person.
Environments that support black-legged/deer ticks are at risk of harbouring B. burgdorferi. In North America, these species of ticks are widely distributed throughout the eastern and midwestern United States. Recent geographic expansion to the north is increasing the prevalence of Lyme disease in Canada.
The increase of human Lyme disease cases highlights the failure of existing preventive strategies — such as minimizing exposure to tick habitats, performing diligent tick checks, and wearing suitable clothing when performing activities in known tick habitats — and emphasizes the need for an effective human vaccine.
A One Health approach
At Vaccine and Infectious Disease Organization at the University of Saskatchewan, we are taking a One Health approach by recognizing that human health is closely related to the health of animals and the shared environment. We are investigating the role of B. burgdorferi, ticks, and susceptible animals on the spread and survival of the Lyme disease bacterium.
It is important to mimic the natural infectious cycle as much as possible when identifying potential vaccine and drug targets. This is because the way host animals are infected (for example, artificial needle infection or natural tick bite) can produce drastic differences in the resulting infection.
Additionally, despite the prevalence of this disease, there are still many aspects of the infectious cycle that remain unknown due to the uniqueness of B. burgdorferi and a lack of knowledge about the tick vector.
For example, we recently learned that a B. burgdorferi protein is responsible for regulating the components necessary for the bacterium to infect vertebrates, including humans. The absence of this protein, among other things, leads to the death of B. burgdorferi in ticks, making it an exciting target for research investigation.
By learning more about the molecular mechanisms that change or reduce the severity of the disease caused by this bacterium, we can identify new targets for the prevention of human Lyme disease.
Jenny Wachter does not work for, consult, own shares in or receive funding from any company or organization that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.
This article was originally published on The Conversation. Read the original article.







