
India has banned the manufacture and export of two highly addictive opioids – tapentadol and carisoprodol – after a report exposed their role in fueling a drug crisis in West Africa.
Aveo Pharmaceuticals, based in Mumbai, was found to be illegally exporting this combination of drugs to countries like Nigeria, Ghana and Ivory Coast.
The BBC secretly recorded footage showing an Aveo director admitting that their drugs were “very harmful” but profitable.
The company’s factory was subsequently raided and its stock seized.
The Indian health ministry announced on Sunday it had started the process to immediately withdraw export and manufacturing licences for the drug combination.
It also suspended all operations by Aveo following an audit by the Central Drugs Standard Control Organisation and the Maharashtra state regulatory authority.
“Approximately 1.3 crore tablets and 26 batches of Active Pharmaceutical Ingredients of tapentadol and carisoprodol were detained to prevent further distribution of these potentially dangerous drugs,” the ministry said.
India’s Food and Drugs Administration vowed stricter monitoring to prevent future illegal exports and safeguard the country’s reputation as a major player in the pharmaceutical industry.
The Drugs Controller General, Dr Rajeev Singh Raghuvanshi, said he had withdrawn permission to manufacture and export the combination due to the “potential of drug abuse and harmful impact on population”.
The Independent has reached out to Dr Raghuvanshi for comment.
“Aveo has been on our radar for the past few months. We served them notice in October for non-matching of their manufacturing and distribution records,” an unnamed official from the Maharashtra drug regulator was quoted as saying by The Times of India paper. “The Central Drugs Standard Control Organization is responsible for testing the exports as per their protocols, and the state does not play a role.”
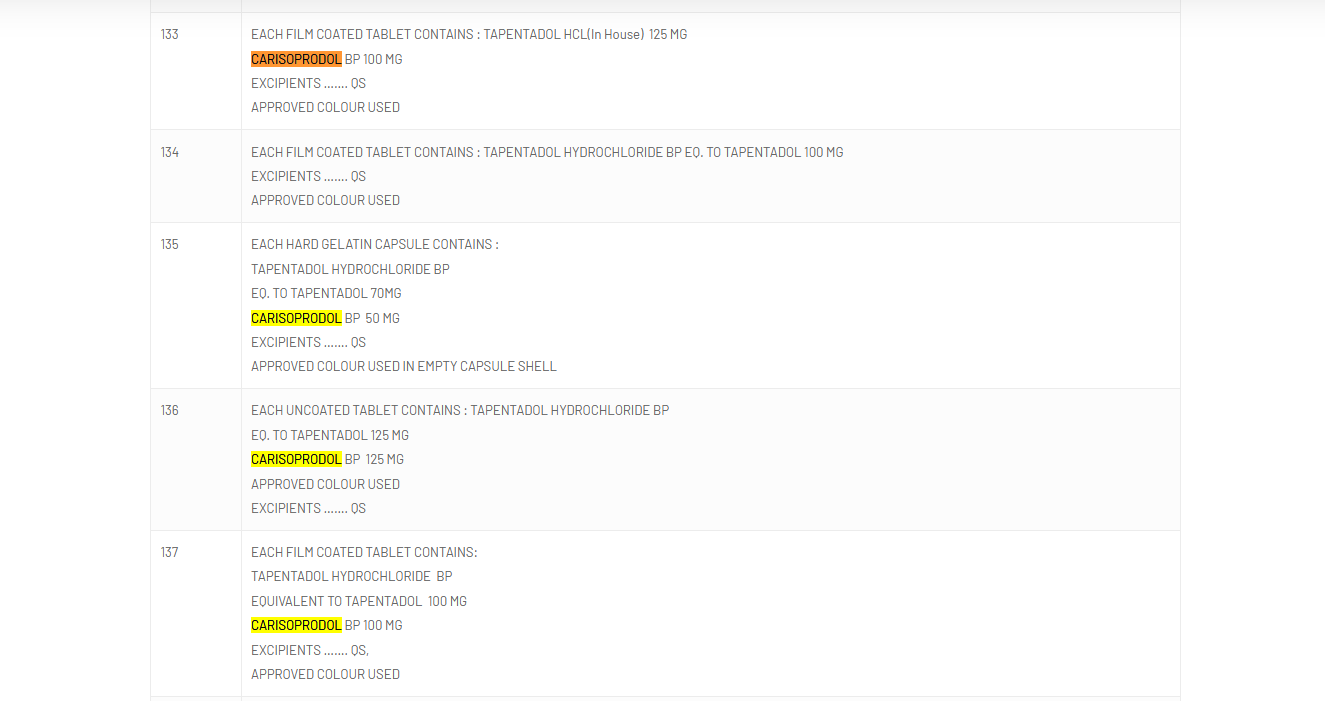
The combination of tapentadol, a powerful opioid, and carisoprodol, an addictive muscle relaxant, is not approved for medical use due to its severe health risks, including breathing difficulties, seizures, and fatal overdoses.
“The medicines have their use separately but their combination is harmful, and it is already banned in India,” the state official explained to the daily.
But the drug combination has flooded West African markets, particularly Nigeria, where an estimated four million people abuse opioids. The World Health Organization estimates that around 100,000 people in Africa die each year due to “falsified or substandard” medication.
The BBC investigation found that Aveo and its sister company, Westfin International, had shipped millions of these pills to West Africa, where they were openly sold on the streets.
In the secretly recorded exchange, Aveo director Vinod Sharma acknowledged the dangers of the pills. “This is very harmful for the health,” he said. “Nowadays, this is business”.
When an undercover BBC operative, posing as an African businessman, suggested selling the pills to Nigerian teenagers, Mr Sharma replied, “OK.”
He explained that taking two or three pills at once would allow users to “relax” and get “high”.
In a statement on its website, Aveo rejected the allegations levelled against it as “entirely baseless and without merit”. “We have always adhered to the rules and regulations set by various regulatory authorities to manufacture and export our products,” it said. “Tafrodol is our registered trademark, which contains both tapentadol and carisoprodol. This combination is licensed by the relevant State Food and Drug Administration and is exported under the necessary No Objection Certificate from the Assistant Drug Controllers and with an export licence issued by the Central Drugs Standard Control Organization.”
The national drug regulator, however, clarified that while tapentadol and carisoprodol were approved for use in India individually, their combination was not.
“It is important to note that Aveo Pharmaceuticals isn’t the only company in India manufacturing a similar combination product. In fact, several companies are unlawfully using our brand name and logo. We have already filed multiple legal cases against such companies, and the matter is currently being heard in the High Court,” a spokesperson for Aveo Pharmaceuticals said. “Furthermore, the manufacturing code of tafrodol blister shown in the BBC article does not bear our manufacturing code of our factory depicting it has been manufactured by some other company.”







