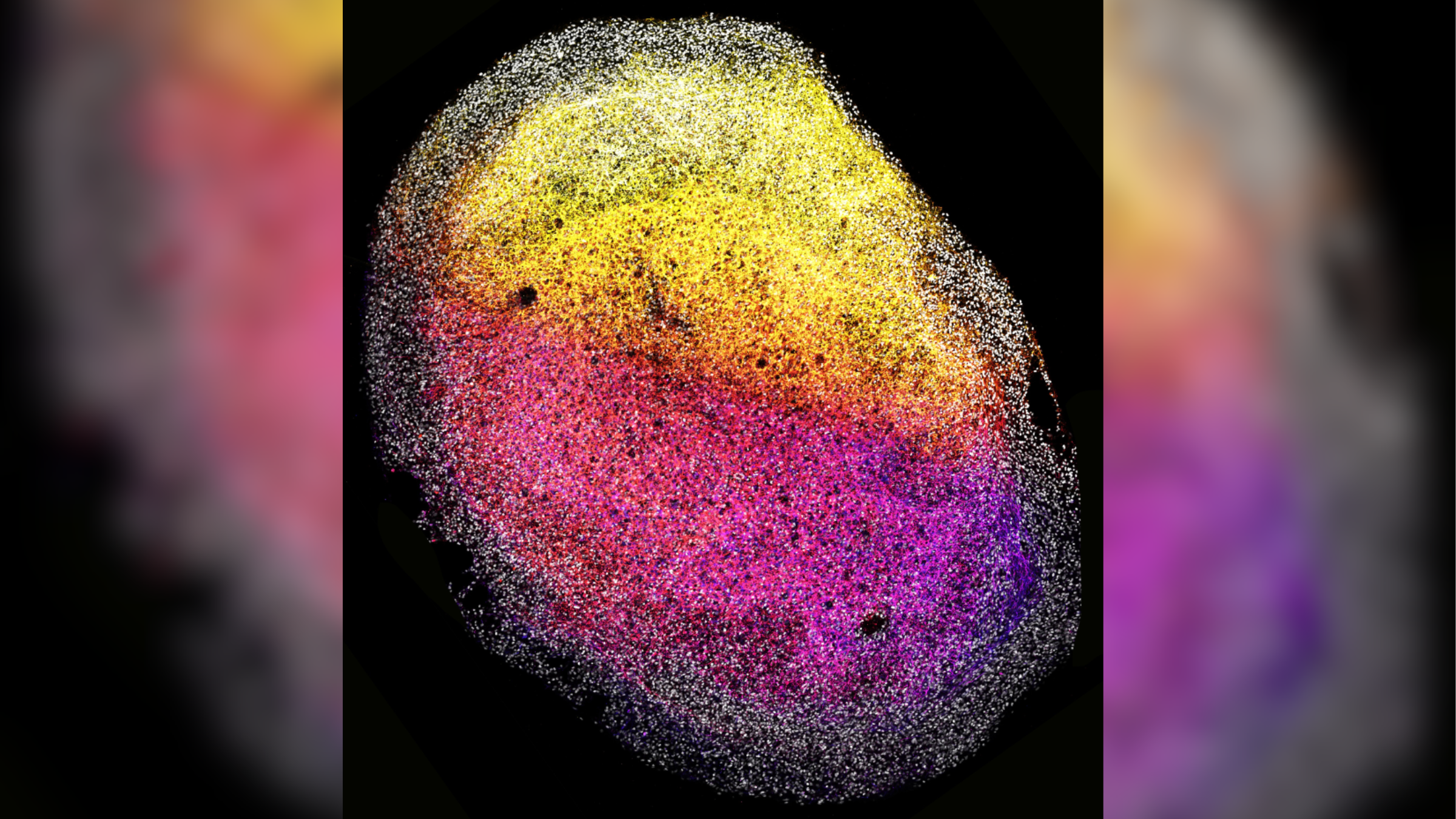
For the first time, scientists have grown cerebral organoids — three-dimensional, lab-grown "minibrains" — from human fetal brain tissue.
The new organoids grew to the size of a grain of rice and contained many types of cells that self-organized into complex 3D structures. The researchers also triggered the growth of brain tumors within the minibrains and tested the tumors' response to existing cancer drugs.
Minibrains mimic key aspects of the development and function of full-size human brains. They have previously been grown from human stem cells but never directly from already formed brain tissue, so the new organoids open up exciting possibilities, the authors of the new study, published Monday (Jan. 8) in the journal Cell, said in a statement.
"Until now, we were able to derive organoids from most human organs, but not from the brain — it's really exciting that we've now been able to jump that hurdle as well," Dr. Hans Clevers, co-senior study author and professor of medicine at Utrecht University in the Netherlands, said in the statement.
The minibrains could complement existing stem-cell-derived organoids and facilitate new, unique ways to study brain health and disease, the researchers said. While stem cells must be coaxed into resembling different parts of the brain, tissue plucked straight from the brain can precisely capture its tissues at specific developmental stages, the authors wrote in the report. And while stem cells must often be provided "scaffolding" to grow upon, brain tissues can make their own, they added.
Thus, "these new fetal tissue-derived organoids can offer novel insights into what shapes the different regions of the brain, and what creates cellular diversity," first study author Delilah Hendriks, an affiliated group leader from the Princess Máxima Center for Pediatric Oncology in The Netherlands, said in the statement.
Scientists have previously engineered minibrains using pluripotent stem cells — primitive cells that have the potential to become any type of cell if supplied with a specific cocktail of chemicals. These minibrains are useful for studying development and associated disorders. However, minibrains derived directly from brain tissue may provide fresh insights into the behavior and characteristics of cells that are specific to the brain, the study authors wrote in the paper.
To make the new minibrains, the researchers took samples of brain tissue from deceased fetuses around the gestational age of 12 to 15 weeks old, which had been provided by anonymous donors. They separately grew small samples of each of the tissues on small plates using specific nutrients and growth factors. Each sample was continuously shaken as it grew, to ensure that all the cells within them were exposed to these chemicals, and they were not provided any physical scaffolding to grow upon.
Around four to eight days later, the researchers noticed the formation of "multiple organized 3D structures" that later matured into organoids with a "tissue-like appearance."
The resulting minibrains contained many types of cells, including neural stem cells known as outer radial glia. The fetal tissues also produced their own extracellular matrix proteins, which acted as scaffolding and supported their 3D organization. This may enable scientists to further study how the environment of cells influences their development.
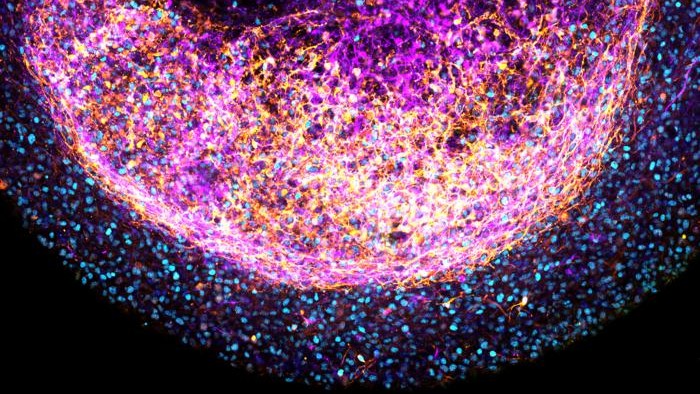
Minibrains grown from certain brain tissues, such as the forebrain, retained structural characteristics specific to that part of the organ, and they responded to specific signaling molecules that are involved in brain development.
In this initial experiment, the minibrains were grown for six months. Notably, the researchers were able to grow multiple minibrains from a single tissue sample. Organoids are emerging as a new way to test the safety and effectiveness of drugs, so this ability to produce many organoids from one sample could someday be useful for conducting repeat experiments of new therapies for disease.
Going forward, the authors want to create more complex versions of the minibrains and potentially develop them from fetal tissues at different stages of development, as well as diseased tissues that could capture brain defects associated with infant mortality.
Like other models of the human brain, "dedicated ethical frameworks" and "active discussions with donors and the scientific community" are needed before any further experiments are conducted using these organoids, the authors wrote in the paper. For instance, researchers must carefully consider the ethics of transplanting human brain tissue into other species, such as rodents, as has been done with stem-cell derived organoids.
Ever wonder why some people build muscle more easily than others or why freckles come out in the sun? Send us your questions about how the human body works to community@livescience.com with the subject line "Health Desk Q," and you may see your question answered on the website!







