This article is part of a fortnightly column exploring contemporary concepts and issues in genetics.
The ‘black death’, or the Great Plague, of the 14th century, was one of the deadliest epidemics in human history. It’s a clear example of the profound influence infectious disease outbreaks can have on society, economy, and culture. It was also probably one of the most impactful epidemics, considering it left an indelible mark on humankind and shaped the collective memory of many subsequent generations.
The ‘black death’ is believed to have killed more than 25 million people in Europe and possibly up to 40-50% of the population in some of the continent’s major cities.
What is the ‘black death’?
The ‘black death’ was caused by a bacterium called Yersinia pestis, which infects mammals. This bacteria’s discovery has been attributed separately to Alexandre Yersin, a Swiss-French physician, and Kitasato Shibasaburō, a Japanese physician and microbiologist during the plague outbreak in Hong Kong in 1894. Humans typically get infected through fleas or through close handling/contact with an infected human or animal.
One possible reason for the humongous proportions of the ‘black death’ outbreak is the human-to-human transmission of the bacteria. While the plague remains a serious disease today, it’s also quite treatable. After the discovery of antibiotics, in fact, its modern mortality is quite small.
Also Read | Where did the Black Death pandemic originate?
India has experienced plague epidemics of varying intensities from as early as 1896 in Bombay to outbreaks in Karnataka (1966) and Surat (1994) and to a more recent isolated outbreak (2004) in a village in Uttarakhand. India also prominently figures in the history of the plague. The plague vaccine was developed by Waldemar Haffkine in 1897 during the outbreaks in Bombay; the country also initiated mass vaccination programmes, with at least 20 million doses estimated to have been administered to date.
How do we study the history of a disease?
While the ‘black death’ is probably not the earliest recorded epidemic, there are old records of its occurrence. Historical archives suggest the Plague of Justinian in the sixth century A.D. was possibly the first to be documented. Plague epidemics continue to occur around the world and are today endemic in some regions.

The evidence also suggests that plague outbreaks were possibly common in Asia and Europe as early as the Late Neolithic-Early Bronze Age (LBNA), as implied by genetic material isolated from a Swedish tomb dated to 3000 BC.
The LBNA period is estimated to have lasted 5,000-2,500 years before present. This era was also characterised by human contact, exchange across Europe, and a consequent social, economic, and cultural transformation of human society.
The advent of genome-sequencing technologies has allowed scientists to trace the trail of infectious diseases that ailed people in prehistoric times. This is possible in particular due to deep-sequencing of genetic material isolated from well-preserved human remains, with the help of advanced computational analysis. Deep-sequencing involves sequencing the genomic material multiple times to retrieve even small amounts of DNA, since the material is likely to degrade over time.
What has deep-sequencing revealed?
Scientists have also traced the prehistoric trail of many major human pathogens in recent years, providing an unparalleled view of the evolution and adaptation of human pathogens.
Consider, for example, a paper published in iScience on May 2. Researchers screened more than 500 tooth and bone samples for genetic material corresponding to Yersinia pestis. They identified five human individuals from whom the genetic material could be isolated, and constructed the genome of the pathogen with deep-sequencing.
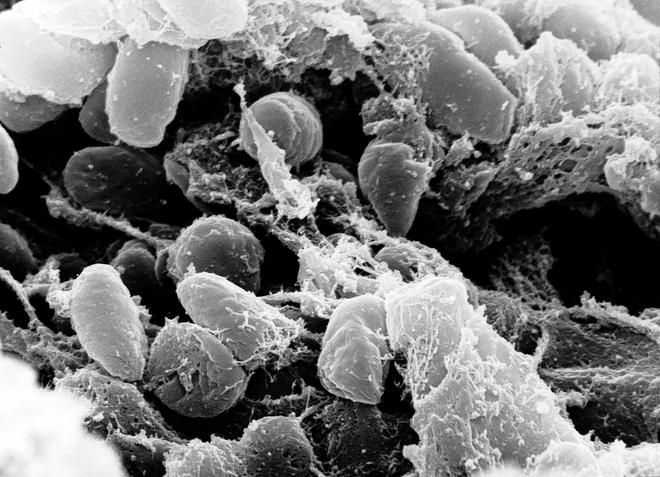
They found that the reconstructed genomes lacked the gene to create a molecule called yapC, short for ‘yersinia autotransporter C’, associated with the bacteria’s ability to bind to mammalian cells and form biofilms – and thus important for causing infections. They also didn’t find the gene for ymt, short for ‘yersinia murine toxin’, which is required for the bacteria’s transmission through fleas. However, they also found the presence of a functional urease D gene, which could make them toxic to fleas.
In another recent paper, published in Nature Communications on May 30, researchers at the Francis Crick Institute, London, reported sequencing genetic material from two distant burial sites in the U.K. They studied 34 human remains in the Charterhouse Warren in Somerset and a ring cairn monument in Levens Park, Cumbria. The remains were estimated to be around 4,000 years old, overlapping with the LBNA period. They identified the genetic material for Yersinia in three individuals, confirming the presence of epidemics in Britain in the LBNA, widening the geographical spread of infections well beyond Eurasia.
What does this teach us about our past?
The genome sequences from the latter also lacked the yapC and ymt genes, reinforcing the previous findings that the plague in that period was possibly not transmitted through fleas.
Indeed, the earliest isolates of Yersinia pestis with the ymt gene, and thus adapted for flea transmission, were possibly from samples from Russia and Spain estimated to be around 3,800 years and 3,300 years old, respectively. The LBNA lineage of Yersinia is therefore believed to have been brought to Europe by the migration of humans from the Eurasian grasslands.
The broad geographical spread over a long timespan also suggests that the plague was possibly quite transmissible in the past, though we know very little of its severity.

As genome-sequencing has become more democratised, its applications are increasingly enabling fast, efficient diagnosis of outbreaks, in routine clinical settings as well, quickly replacing the traditional approaches in microbiology. Sequencing provides enormous advantages over conventional approaches because it can contribute to identification and molecular characterisation, and open windows into virulence, antimicrobial and antibody resistance, and clues into the evolution, adaptation, and introduction of species in new settings.
The ambit of such technologies is also expanding to include studies of animal and plant diseases, along with human diseases, contributing to the unified understanding of our well-being called ‘One Health’.
Sridhar Sivasubbu and Vinod Scaria are scientists at the CSIR Institute of Genomics and Integrative Biology (CSIR-IGIB) . All opinions expressed are personal.







