
It was time for Dr. Musavi to convey the sad news. With her health rapidly declining, his patient, Mrs. Ozra, had less than two weeks to live. Summoning the family together, he encouraged them to make arrangements.
Although it was obvious to the clinician, this news came as a surprise to the family. Ezra’s oldest son, Arman, had been living in exile for the last 20 years, and it would be hard for him to return to say goodbye. They begged the doctor to do all he could to extend her life until they could get Arman home. Musavi paused for a moment.
“There is a new treatment that I could try,” he said. “It’s been having some promising results. I could try it on your mother.”
With hope and relief, the family gave Musavi permission to proceed.
It took over a month for the family to get Arman home. By the time they brought him to his mother, the transformation was evident. Ozra had a surprising return to health. When she passed over a year later, the family contacted Musavi to thank him for the lifesaving treatment.
“Can I tell you a secret?” he asked, in a hushed tone. “It was only sugar cubes.”
Placebo potential

What if what you expect to experience from a treatment influenced how well the treatment works? If this were true, then past experiences, observations of the experience of others, and verbal suggestions could also influence your symptoms. And what if you were unaware that the treatment was inert, that it was a placebo? Would your experience of the treatment’s effects be more like the treatment you think you are getting or like a placebo?
There is considerable experimental evidence that expectations drive our experience of treatment regardless of whether the treatment is a drug or placebo. When the treatment is inert, the experience of benefit is called a placebo effect. A popular model of how placebo effects work proposes that expectations lead to psychophysiological effects, which in turn reduce symptoms. In addition to expectation, placebo effects can be shaped by associative learning. Associative learning or conditioning is the process by which a symbol (i.e., a bell or pill) is repeatedly paired with a stimulus (i.e., food or pain reduction). As a result, the symbol or cue alone elicits the effect of the original stimulus.
Conditioning and expectation are not separate processes but rather represent a continuum by which information is processed and used consciously or unconsciously by an individual to guide perception and response.
Associative learning
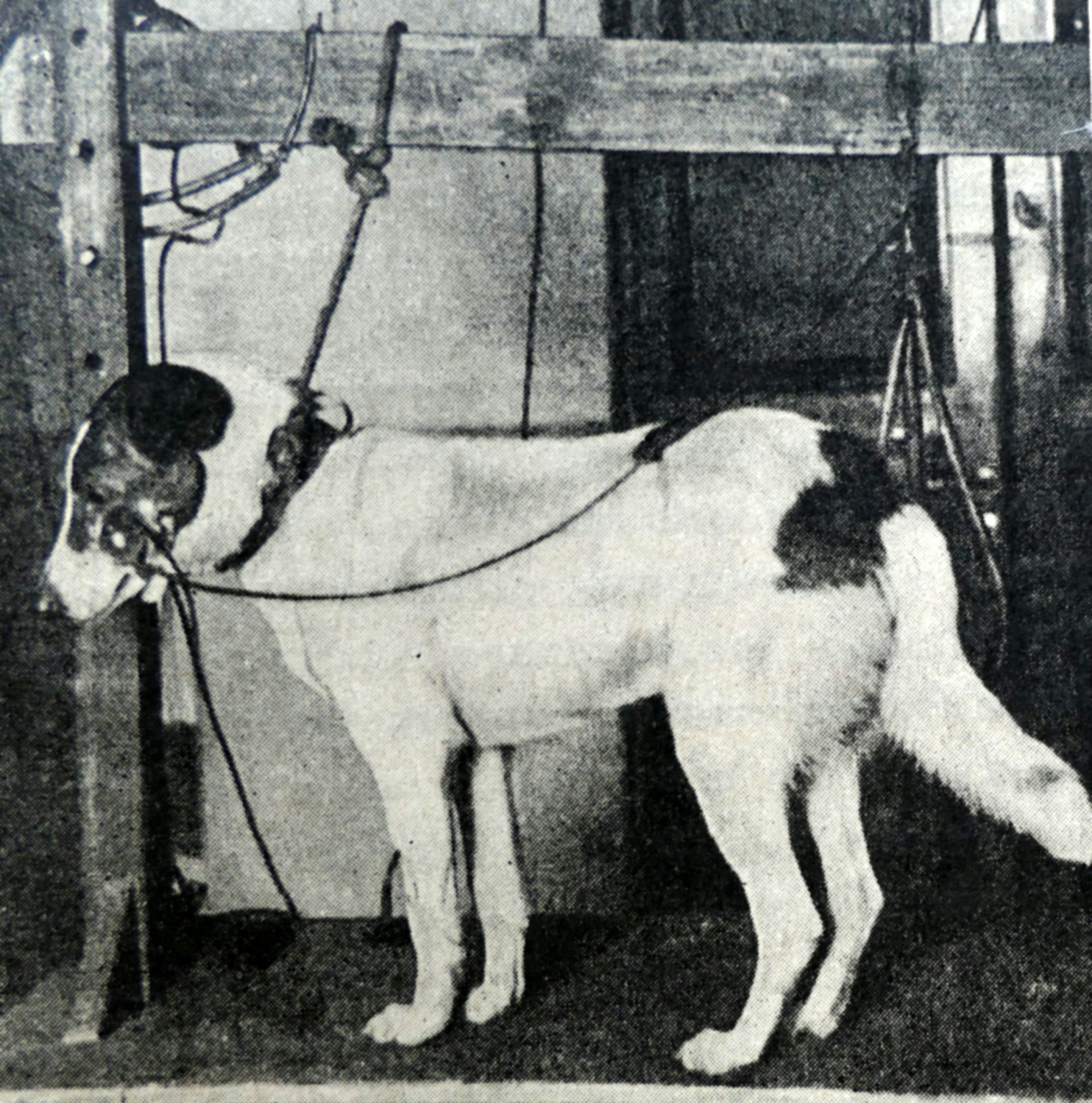
Classical conditioning, the process of training or habituating a human or animal to respond to a stimulus in a certain way, derives from the methodology laid out by the Russian physiologist Ivan Petrovich Pavlov in the early 1900s. In his famous experiment, Pavlov repeatedly paired the sight and smell of food, which naturally causes dogs to salivate, with an unrelated neutral but novel event like a buzzer or metronome (not a bell, as commonly believed, according to Pavlov biographer Daniel Todes). After a number of repeated pairings, the sound alone induced salivation independently of the presence of the sight or smell of food. Although the connection between Pavlov’s finding to placebo effects is patently clear in hindsight, it would take another half century before we made the leap to conditioning drugs in humans.
In 1962, Richard J. Herrnstein (who would co-author the controversial The Bell Curve 30 years later) reported the conditioning of placebo effects in rats. By repeatedly pairing saline injections with scopolamine, a drug that depresses muscular movement and coordination, Herrnstein was able to depress movement in rodents by saline injection alone. In subsequent conditioning experiments, other researchers demonstrated that animals could be conditioned with other drugs like morphine or amphetamine. But not every drug had the expected effect.
In 1971, Robert Pihl and Jack Altman paired saline with the tranquilizer Thorazine. Instead of conditioning depressed activity, the rats were more active than ever with the subsequent saline injections. Pihl and Altman reasoned that there is likely an interaction between the nature of the drug being studied and the response being measured. Thorazine inhibits dopamine signaling in the region of the brain involved with learning. Could Thorazine be an exception to the conditioning paradigm because it blocked the acquisition stage of learning? Could Thorazine be one of those drugs like naloxone that can perturb a placebo response?
While exceptions to the conditioning paradigm remained a curiosity, studies rapidly progressed to conditioning the immune function. As with many important discoveries in science, the finding that immunosuppression could be conditioned in animals occurred by chance through taste aversion studies in rodents dating back to the 1940s. The British Army, trying to eradicate rodents from foxholes during World War II, observed that rats would sparingly sample poisoned food and then assiduously avoid subsequent exposures to it. Rats are unable to vomit to purge themselves of toxic substances, and hence taste aversion is an important adaptation that helps them avoid drinking or eating potentially noxious substances. Taste aversion is rapid and persists for over a month, and thus provided researchers with an excellent animal model for studying associative learning with other drugs like lithium chloride, morphine, and cyclophosphamide.
Early studies pairing saccharin with cyclophosphamide, an immunosuppressant drug, found that many of the conditioned animals died when they were rechallenged with saccharin. As it was well known that saccharin did not kill rodents, these findings suggested that the conditioning protocol caused an overactive immunosuppressant response to the saccharin, which in turn led to the demise of the animals.
In 1975, Robert Ader and Nicholas Cohen followed up on these initial findings and demonstrated that indeed pairing a drinking solution of saccharin with an injection of the immunosuppressant cyclophosphamide could induce immunosuppression when the animals subsequently drank saccharin alone. This study inspired a series of experiments in which specific immune function changes, like the reduction in white blood cell count, weight reduction in the spleen and thymus, and changes in cytokines, could all be conditioned by varying the specific immunosuppressive drug. The inverse was also true. The enhanced immune function could be conditioned by pairing saccharin with drugs that activate white blood cells.
Immune modulation in animals was soon translated to humans. In 1992, Angelika Buske-Kirschbaum and colleagues paired sherbet with an epinephrine injection, which is known to elicit an increase in natural killer cell activity. When given sherbet with saline injection, the conditioned group showed an increase in natural killer cell activity. In subsequent years, the conditioning of placebo effects became an established protocol.
Today, researchers are actively exploring using conditioning, or as it is currently termed, associative learning, to reduce the use of opioids. In these “dose-extension” studies, placebo pills are interspersed with verum opioids, and through associative learning, the dose of pain treatments can be gradually reduced and replaced with a placebo.
Although compelling as the mechanism by which placebos mediate their effects, classical conditioning falls short of explaining key features of placebo effects. In the clinical setting, the response to a placebo can be induced by less deliberate processes than conditioning. Further, potent pharmacological drug effects can be modified by suggestion. For instance, the pain-killing effects of morphine are substantially reduced when its administration is hidden from the patient.
For a while, there was a robust debate regarding the difference between conditioning and expectancy. This debate, however, resolved with the understanding that both expectation and conditioning require some level of association between the “inert” (e.g., a placebo pill) and “active” (e.g., a drug) stimulus that requires cognitive processing. After all, Pavlov had to pair the smell and sight of food with the buzzer, or he could ring the bell till the cows came home, and nobody would salivate.
Great expectations
One of the most striking examples of how expectation can influence symptom relief is the mirror cure for phantom limb pain. The amputation of an arm or leg can leave many patients still experiencing the limb as if it were present, and sometimes this causes extreme pain in the “phantom limb.” In 1992, Vilayanur Ramachandran developed a simple and ingenious treatment. He placed patients beside a mirror box in which they could see their remaining limbs reflected in the mirror. This gave the patients the impression that they were viewing their real and healthy original limbs.
While looking at the image of their hand in the mirror, the patients were instructed to send movement commands to both of their limbs and make symmetrical movements that one would typically make with both limbs. In the case of an amputated hand, they could make the motions of conducting a band or opening and closing both hands. Quite miraculously, as they watched “both their hands” seemingly functioning normally, they experienced an amelioration of the pain. Thus mirror visual feedback (MVF), as this procedure is called, builds an experience of their phantom hand as functional, pain-free, and able to enact commands that shift the expectation of how their hands feel. MVF, which is remarkably effective at ameliorating phantom limb pain, is by no means inert and is not formally considered a placebo treatment, but this therapy underscores the power of our perceptions in having a hand in modulating our symptoms.
Expectations can relieve as well as induce pain. In 1995, a 29-year-old builder was rushed to the emergency department at Leicester Royal Infirmary after jumping down onto a 7-inch nail that went right through his boot. He was sedated with midazolam and fentanyl as the slightest movement of the nail was extremely painful. After carefully removing the boot, it was immediately apparent that while the nail had entered proximal to the steel toe cap, it had slid between the builder’s toes, and his foot was entirely unharmed. This information immediately relieved the man’s pain.
A predictive path

The term expectancy is common in the placebo literature and refers to the unconscious predictions that individuals hold. Just as Ozra expected to benefit from Musavi’s treatment, and the builder expected pain from the 7-inch nail in his boot, expectations in the context of a placebo (response expectancy) are theorized to elicit the suggested or believed response because the subject expects it to do so. Expectations are shaped by learning from past experience, informed by contextual verbal and nonverbal cues, and can be either positive or negative. Thus through placebo mechanisms, expectations can enhance or minimize the effects of treatment.
The effects of expectation on pharmacologically active treatments were elegantly demonstrated in a study in which Ulrike Bingel and colleagues used three different conditions with verbal instructions to manipulate the effects of the powerful painkiller remifentanil. Remifentanil is a fast-acting opioid analgesic administered by infusion and used to manage pain after surgery. In this experimental setting, healthy individuals were hooked up to an infusion pump and asked to rate a painful thermal stimulus that was administered before and during the infusion at various time points. Throughout the experiment, the information given to the participants about the infusion was changed to modulate expectancy, but the level of the thermal heat pain stimulus remained the same across the whole experiment.
At the baseline, when the saline infusion was initiated, the participants were asked to rate their pain on a scale of zero to 100. The average pain rating at the baseline was 66. Without the awareness of the participants, the remifentanil infusion pump was then turned on and run for the duration of the experiment. After 30 minutes of “hidden” remifentanil infusion, the second thermal pain stimulus was administered. Again the participants were asked to rate their pain. This time, they rated their pain slightly lower, at around 55, suggesting only a small benefit from this potent drug. The investigator then told the participants that the infusion was about to begin, even though it had already started. With this “open” remifentanil infusion, a positive expectancy was created. When, once again, the same pain stimulus was administered, the participants rated their pain to be much lower, at around 39. Hence there was significantly less pain experienced with “open” compared to “hidden” remifentanil. Finally, the participants were told that the infusion was being stopped and they might expect an increase in pain with the next thermal stimulus. Although the infusion was not stopped, when once again they got the same pain stimulus, as a result of negative expectancy, they now rated their pain significantly higher, at about 64.
The pain rating with this second “hidden” remifentanil condition was almost back to the baseline level before the remifentanil infusion was initiated. The researchers also measured unpleasantness and anxiety and found similar results to the pain ratings. By concealing the identity of the infusions, Bingel and colleagues were able to isolate the effect of verbal information and visual cues on pain and unpleasantness ratings and demonstrated that these cues modulate treatment outcomes significantly. While positive expectancy dulled the participants’ pain, negative expectancy actually exacerbated pain, in spite of the powerful analgesic.
The Bingel study was not the first to document the malleability of pharmacological effects; it was preceded by numerous studies that demonstrated that verbal suggestion could induce the opposite of the expected effects. For example, the verbal suggestion could reverse the analgesic effects of nitrous oxide, elicit sedative effects with epinephrine, amphetamine, and aspirin, and induce stimulating effects with the sedative chloral hydrate. Using the open-hidden paradigm, with informed consent, the effects of powerful drugs like diazepam were reduced by 50 percent if the administration was hidden. Similarly, when the administration of the drugs buprenorphine, tramadol, ketorolac, and metamizole was hidden, 50 percent more drug was needed to elicit the typical analgesic effects observed when these drugs were administered openly.
The labeling of drugs can also have a strong influence on expectations and, consequently, treatment outcomes. A good example of this is the migraine study done by Slavenka Kam-Hansen and colleagues comparing the effects of labeling a placebo and Maxalt (the brand name of rizatriptan, an effective headache medicine that works by narrowing the blood vessels in the brain). In the study, matching bottles containing a placebo or Maxalt were labeled as “placebo,” “Maxalt,” or “placebo or Maxalt.” Thus the bottles were either correctly, incorrectly, or ambiguously labeled. The order in which the patients were supposed to use the pills from each of these bottles was randomized for each participant; for the next six migraine attacks, they would use pills from the bottles in the order they were assigned. The patients were asked to report their level of pain 30 minutes after the onset of a headache (the baseline) and then take study pills from the designated bottle. Two and half hours after the headache onset, they were asked again to record their level of pain. After 459 headaches, the results were in. Maxalt labeled as Maxalt was the most effective at treating headaches, but there was no difference statistically between Maxalt mislabeled as a placebo and a placebo mislabeled as Maxalt.
One gets what one pays for

The influence of expectations on treatment outcomes is not limited to explicit manipulations of information in the clinical encounter. Studies examining manipulations of cost, branding, and subtle cost-related cues have found that patients hold subconscious associations between cost, branding, and treatment efficacy that influence treatment outcomes. Study participants who receive a treatment that “costs more” tend to experience a greater benefit as compared to when they receive a treatment that “costs less,” even when the treatments are identical and inert. Even switching between brands, especially from a name brand to a generic, has been shown to modify subjective and objective outcome measures of efficacy.
Expectation-related effects can also be transmitted by social observational learning. Social observational learning occurs when the observation of a demonstrator’s behavior modifies the behavior of the observer. When combined with other nonverbal cues, conditioned social learning can enhance placebo responses. This effect can cause negative responses too; a good example of this phenomenon in clinical trials is the increased likelihood of patients experiencing side effects after hearing about them from other patients, even if the patients are taking a placebo. These nocebo effects are somewhat common in clinical trials. In an influential study, patients were randomized to placebo or montelukast, an active treatment for asthma, with neutral or positive treatment expectations. Strikingly, participants given literature that mentioned headaches as a possible side effect of montelukast experienced headaches more frequently, even if they were taking a placebo.
Given the influence of expectations on clinical outcomes and side effects in a wide cross-section of studies and conditions, more attention could be paid to the information that patients are exposed to during clinical trials. On the one hand, there is an ethical imperative in shared decision-making between the patient and clinician that calls for the patient to be fully informed about the potential benefits and risks involved in a study. On the other hand, the very act of informing the patient about the benefits and risks of the study shapes the benefits and risks of the study. How to balance these competing ethical imperatives to provide what is true of benefit to the patient remains in question.
This article was originally published on The Reader by Kathryn T. Hall. Read the original article here.







