Medical laboratory testing is the heartbeat of medicine. It provides critical data for physicians to diagnose and treat disease, dating back thousands of years. Unfortunately, laboratory medicine as a field is poorly understood by both the public and health care communities.
Laboratory medicine, also known as clinical pathology, is one of two main branches of pathology, or the study of the causes and effects of disease. Pathology covers many laboratory areas, such as blood banking and microbiology. Clinical pathology diagnoses a disease through laboratory analysis of body fluids such as blood, urine, feces and saliva. The other branch of pathology, anatomic pathology, diagnoses a disease by examining body tissues.
We are public health and medical laboratory scientists who specialize in microbiology and infectious diseases. There are a lot of steps between when your doctor orders a blood test to establishing a diagnosis. From the bedside to the lab bench, here’s how laboratory testing works.
It all starts with a specimen
When you see a doctor, sometimes a physical exam and detailed medical history are enough for them to make a diagnosis, offer recommendations or prescribe medications for your condition. There are many instances, however, where your doctor may need additional information to make an accurate diagnosis. This information is often obtained from procedures like imaging scans or blood tests.
The first step involves getting your blood drawn through a practice known as phlebotomy. A health care professional, typically a phlebotomist or a nurse, inserts a needle into a vein to collect a blood specimen.
Multiple tubes of blood may be needed, as certain tests are only performed using certain types of blood specimens. For example, one test commonly used to diagnose anemia requires blood to be collected in a chemical that prevents the blood from clotting. Patients being evaluated for a clotting disorder, on the other hand, often have their blood collected in a tube containing another anticoagulant.
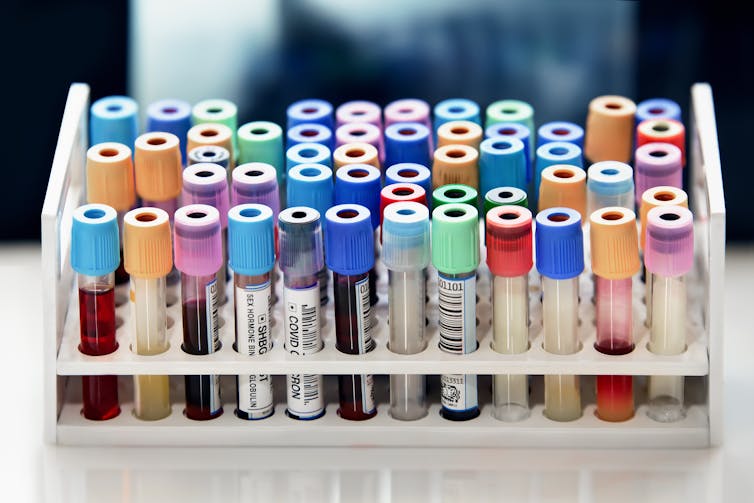
Testing pathways
Specimens then make their way to a clinical laboratory. Laboratories can be found within hospitals, reference labs or physician offices, or they can be located in a public health setting such as the Centers for Disease Control and Prevention or a state public health laboratory. In 2021, there were more than 329,000 laboratory professionals working in the U.S. in more than 320,000 federally regulated laboratories. An estimated 14 billion laboratory tests are ordered annually in the U.S., on top of over 1 billon COVID-19 tests during the pandemic. With such a large volume of specimens to test and examine, various sections of a laboratory are automated.
Laboratory tests examine the biological, chemical and physical properties of the cells and molecules that make up a blood specimen. The first step is often to centrifuge a blood specimen into separate components. This divides the sample into one portion that contains solid components, such as cells, and another that contains liquid components and dissolved solutes, known as serum or plasma.
Analyzing the serum or plasma portion of a blood specimen measures the levels of different substances within the body. One of the most common is your blood sugar, or glucose concentration. For the doctors of more than 37 million Americans with diabetes, knowing how high their patient’s blood glucose is helps them establish a new diagnosis or ensure their condition is under control.
If your doctor suspects you have an infection, they will collect specimens to test for the presence of a pathogen. For example, they might collect a throat swab for strep throat or a urine sample for a urinary tract infection. Scientists incubate these samples to screen any organisms that grow and resemble pathogens of interest. They may perform additional testing to identify the microbe. Once an organism is identified, the medical laboratory professional can then test a variety of antimicrobial agents against it to inform your doctor what the best treatment would be against your infection.
Evolution of medical laboratory testing
The first hospital clinical laboratory in the U.S. was established in 1894. Some of the methods laboratory professionals use to analyze samples have been in use for over a century.
One such staple, the Gram stain, was introduced in 1882. It uses two different dyes and exploits differences in the bacterial cell wall to discriminate between two different groups of bacteria. This helps lab scientists identify the correct antimicrobial therapy to use against an infection.
Another commonly used technology, the Coulter Principle, was developed in the 1940s to identify and sort individual cells based on physical size and resistance to an electrical current. Medical laboratory professionals routinely use this technique to conduct complete blood count tests, which measure unusual increases or decreases in the number of different types of blood cells that could provide insights into a disease or condition, such as cancer or sickle cell anemia.
In 1986, scientists devised the Nobel Prize-winning polymerase chain reaction method to amplify, or rapidly produce, multiple copies of the DNA of a pathogen present within a sample. PCR is widely used to diagnose infections, identify genetic disorders and monitor cancer progression.
An explosion of modern laboratory tools to research and diagnose disease followed PCR. To name a few of these cutting-edge tools, matrix-assisted laser desorption ionization, or MALDI, is one of the most commonly used techniques to identify microbes that are difficult or impossible to culture. Genome editing and CRISPR-Cas9 give scientists the ability to change an organism’s DNA, aiding in identifying pathogens and detecting dysfunctional genes by adding, removing or altering genes of interest. Next-generation sequencing has become a powerful modern tool to determine the sequence of the genetic material in biological samples and has been extensively used to identify variants and wastewater surveillance of pathogens like the virus that causes COVID-19.
Challenges and solutions
One of the most critical challenges in laboratory medicine is understanding and interpreting test results, because errors can occur throughout the testing process. Specimens must be properly collected and transported to the lab for accurate results. Likewise, at-home tests need to be properly stored. Clinicians and patients need to take into account the chances of false positive or negative results by considering the limitations of the test alongside the patient’s individual case.
Collaboration between clinicians and medical laboratory professionals could help reduce errors in diagnosis and treatment. Laboratory data can and often is extremely useful to patient care, but a holistic approach that takes into account a patient’s medical history, genetics and health habits, among other factors, is necessary for an accurate diagnosis and treatment. While powerful, a laboratory result should not be used in isolation. Clear and accurate communication on laboratory testing is critical for effective patient care.
Rodney E. Rohde has received funding from the American Society of Clinical Pathologists, American Society for Clinical Laboratory Science, U.S. Department of Labor (OSHA), and other public and private entities/foundations. Rohde is affiliated with ASCP, ASCLS, ASM, and serves on several scientific advisory boards. See https://rodneyerohde.wp.txstate.edu/service/.
Nicholas Moore previously received funding from Abbott Molecular, bioMerieux, and Cepheid for contracted research work related to the development of laboratory assays. Funds were paid directly to Rush University. Nicholas Moore is a volunteer with the American Society for Clinical Laboratory Science, the American Society for Clinical Pathology, the American Society for Microbiology, and the Clinical and Laboratory Standards Institute. He is a member of the editorial board of Clinical Microbiology Reviews and BMC Infectious Diseases.
This article was originally published on The Conversation. Read the original article.