Gene therapy has been headline news in recent years, in part due to the rapid development of biotechnology that enables doctors to administer such treatments. Broadly, gene therapies are techniques used to treat or prevent disease by tweaking the content or expression of cells' DNA, often by replacing faulty genes with functional ones.
The term "gene therapy" sometimes appears alongside misinformation about mRNA vaccines, which include the Pfizer and Moderna COVID-19 vaccines. These vaccines contain mRNA, a genetic cousin of DNA, that prompts cells to make the coronavirus "spike protein." The vaccines don't alter cells' DNA, and after making the spike, cells break down most of the mRNA. Other COVID-19 shots include the viral vector vaccines made by AstraZeneca and Johnson & Johnson, which deliver DNA into cells to make them build spike proteins. The cells that make spike proteins, using instructions from either mRNA or viral vector vaccines, serve as target practice for the immune system, so they don't stick around long. That's very, very different from gene therapy, which aims to change cells' function for the long-term.
Let's take a dive into what gene therapy actually is, addressing some common questions along the way.
What is gene therapy, and what does it do to your DNA?
DNA is a molecule that stores genetic information, and genes are pieces of genetic information that cells use to make a particular product, such as a protein. DNA is located inside the nucleus of a cell, where it's packaged into chromosomes, and also inside mitochondria, the "power plant" organelles located outside the nucleus.
Although there are mitochondrial diseases that could someday be cured with gene therapy, currently, the term gene therapy refers to treatments that target nuclear genes — the genes on the 23 pairs of chromosomes inside the nucleus.
Classically, gene therapy has referred to the process of either "knocking out" a dysfunctional gene or adding a copy of a working gene to the nucleus in order to improve cell function. Gene therapy is currently directed at diseases stemming from a problem with just one gene, or at most a few genes, rather than those that involve many genes.
However, the field of gene therapy is now expanding to include strategies that don't all fall into the classic categories of knocking out bad genes or adding good genes. For example, researchers at Sangamo Therapeutics are developing genetic techniques for treating Parkinson, Alzheimer and Huntington diseases that work by ramping up or suppressing the activity of specific genes.
While the treatments may add genes to body cells, knock out genes or act in some way to change the function of genes, each gene therapy is directed to the cells of particular body tissues. Thus, when scientists and doctors talk about what gene therapy does to DNA, they are not talking about all of the DNA in the body, but only some of it.
How does gene therapy work?
Gene therapy can be either ex vivo or in vivo.
Ex vivo gene therapy means that cells are removed from the body, treated and then returned to the body. This is the approach used to treat genetic diseases of blood cells, because bone marrow can be harvested from the patient, stem cells from that bone marrow can be treated with gene therapy — for instance, to supply a gene that is missing or not working correctly — and the transformed cells can be infused back into the patient.
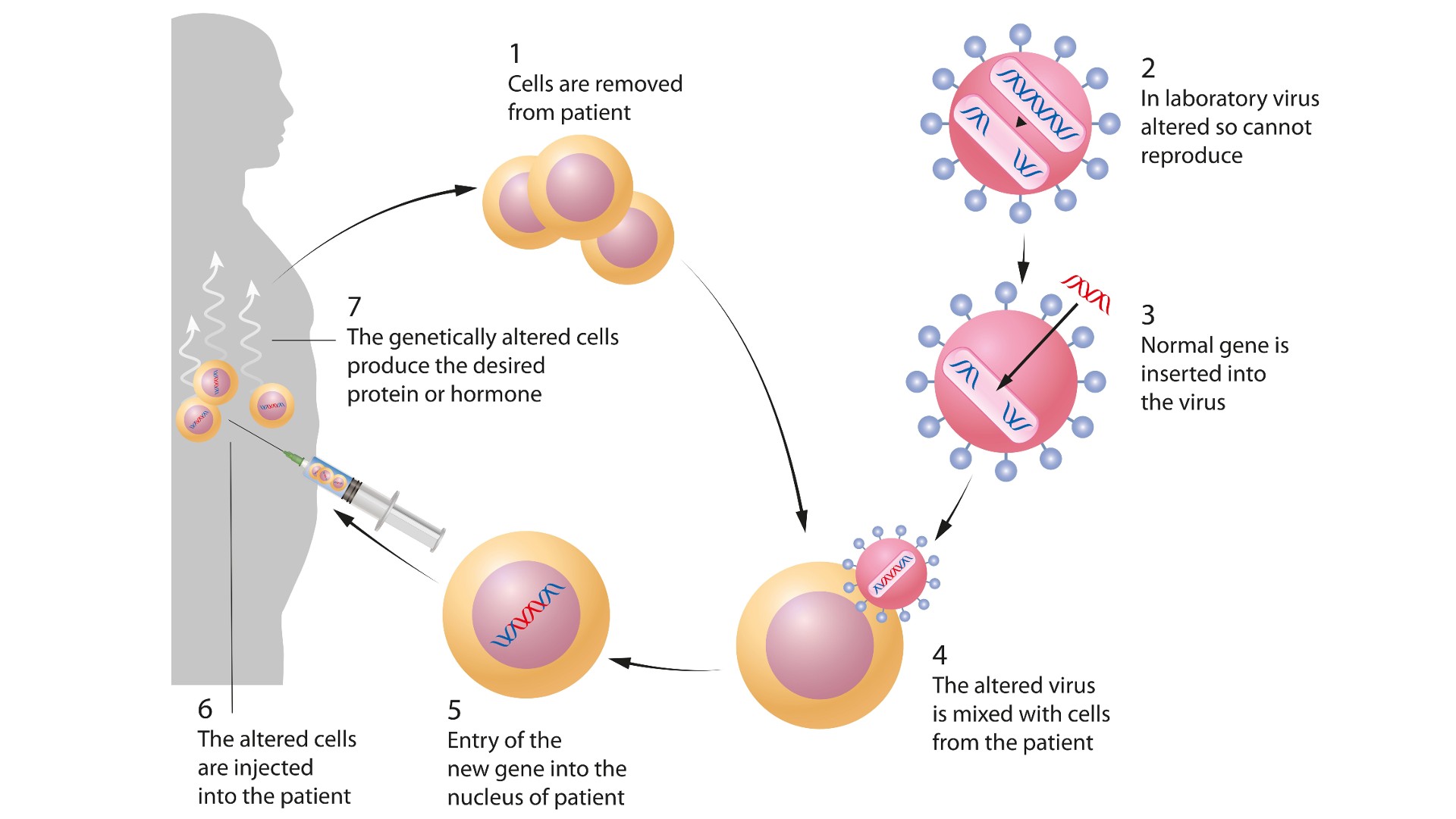
In vivo gene therapy means that the gene therapy itself is injected or infused into the person. This can be through injection directly to the anatomic site where the gene therapy is needed (a common example being the retina of the eye), or it can mean injection or infusion of a genetic payload that must travel to the body tissues where it is needed.
In both ex vivo and in vivo gene therapy, the genetic payload is packaged within a container, called a vector, before being delivered into cells or the body. One such vector is adeno-associated virus (AAV). This is a group of viruses that exist in nature but have had their regular genes removed and replaced with a genetic payload, turning them into gene therapy vectors.
Is gene therapy safe?
AAV has been used to deliver gene therapy for many years, because it has a good safety record. It is much less likely to cause a dangerous immune response than other viruses that were used as vectors several decades ago, when gene therapy was just getting started. Additionally, packaging genetic payloads within AAV carriers allows for injected or infused gene therapy to travel to particular body tissues where it is needed. This is because there are many types of AAV, and certain types are attracted to certain tissues or organs. So, if a genetic payload needs to reach liver cells, for example, it can be packaged into a type of AAV that likes to go to the liver.
In the early days of gene therapy, which began in 1989, researchers used retroviruses as vectors. These viruses delivered a genetic payload directly into the nuclear chromosomes of the patient. However, there was concern that such integration of new DNA into chromosomes might cause changes leading to cancer, so the strategy was initially abandoned. (More recently, scientist have successfully used retroviruses in experimental gene therapies without causing cancer; for example, a retrovirus-based therapy was used to treat infants with "bubble boy disease.")

After moving away from retroviruses, researchers turned to adenoviruses, which offered the advantage of delivering the genetic payload as an episome — a piece of DNA that functions as a gene inside the nucleus but remains a separate entity from the chromosomes. The risk for cancer was extremely low with this innovation, but adenovirus vectors turned out to stimulate the immune system in very powerful ways. In 1999, an immune reaction from adenovirus-carrying gene therapy led to the death of 18-year-old Jesse Gelsinger, who'd volunteered for a clinical trial.
Gelsinger's death shocked the gene therapy community, stalling the field for several years, but the current gene therapies that have emerged over the years based on AAV are not dangerous. However, they tend to be expensive and the success rate varies, so they typically are used as a last resort for a growing number of genetic diseases.
What conditions are currently treated with gene therapy?
Gene therapy can treat certain blood diseases, such as hemophilia A, hemophilia B, sickle cell disease, and as of 2022, beta thalassemia. What these diseases have in common is that the problem comes down to just one gene. This made beta thalassemia and sickle cell disease low-hanging fruits for ex vivo gene therapies that involve removing and modifying bone marrow stem cells, whereas hemophilia A and hemophilia B are treated with in vivo gene therapies that target liver cells. That said, other treatments exist for these blood diseases, so gene therapy is more of a last resort.
Numerous enzyme deficiency disorders also come down to one bad gene that needs to be replaced. Cerebral adrenoleukodystrophy, which causes fatty acids to accumulate in the brain, is one such disorder that can be treated with gene therapy, according to Boston Children's Hospital. CAR T-cell therapy, which is approved for certain cancers, involves removing and modifying a patient's immune cells and is known as a "cell-based gene therapy."
Gene therapy has also been useful in treating hereditary retinal diseases, for which other treatments have not been useful.
What gene therapies are in development?
Another group of targets for gene therapy are diseases of the nervous system.
"We are at a remarkable time in the neurosciences, where treatments for genetic forms of neurological disorders are being developed," Dr. Merit Cudkowicz, the chief of neurology at Massachusetts General Hospital and a professor at Harvard Medical School, told Live Science.
For example, gene therapies are being developed to treat a pair of genetic diseases called Tay-Sachs disease and Sandhoff disease. Both conditions result from organelles called lysosomes filling up with fat-like molecules called gangliosides. The effects of these diseases include delay in reaching developmental milestones, loss of previously acquired skills, stiffness, blindness, weakness and lack of coordination with eventual paralysis. Children born with Tay-Sachs disease and Sandhoff disease generally don’t make it past 2 to 5 years of age.
"There has been no routine antenatal or neonatal test for Tay-Sachs and Sandhoff, because there has been no available treatment whatsoever," said Dr. Jagdeep Walia, a clinical geneticist and head of the Division of Medical Genetics within the Department of Pediatrics and the Kingston Health Sciences Centre and Queen's University in Ontario, Canada. Walia is developing a gene therapy aimed at replacing the gene for Hex A, the enzyme that is deficient in these children. So far, the treatment has shown good efficacy and safety in animal models, but it still needs to be tested in human patients.
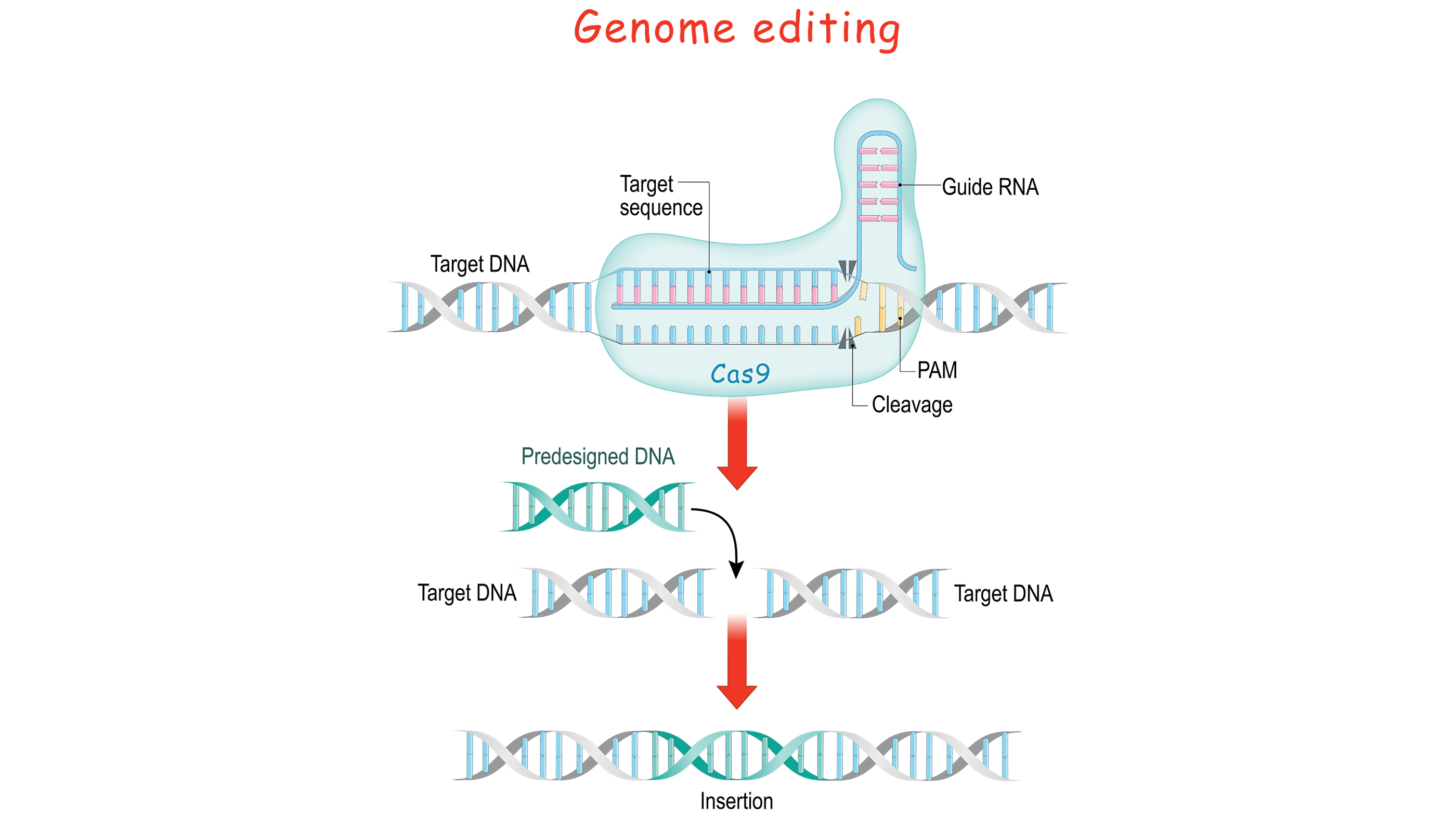
The future looks hopeful when it comes to gene therapy overall, on account of new technological developments, including CRISPR gene editing. This is an extremely powerful technique for cutting out parts of DNA molecules and even pasting new parts in — analogous to what you do with text in word processing applications. CRISPR is not the first method that scientists have used to edit DNA, but it is far more versatile that other techniques. It is not yet quite ready for in vivo chromosomal manipulation, but it is advancing exponentially.
Perhaps even closer to the horizon is the prospect of delivering larger genetic payloads into cells. One big drawback of the AAV vector is that each virus particle can carry just a small amount of DNA, but recent research has revealed that a different type of virus, called cytomegalovirus, can be adapted to carry gene therapies with a much bigger payload than AAV. Not only might this some day expand gene therapy to more diseases requiring larger genes than AAV can carry, but it also could enable more than one gene to be delivered in a single therapy.







