In recent months, 22 people across 11 U.S. states have developed symptoms like blurry vision and shortness of breath after getting "counterfeit or mishandled" Botox injections outside medical settings, such as in spas and people's homes.
In an advisory issued Tuesday (April 23), the Centers for Disease Control and Prevention (CDC) alerted doctors to an ongoing investigation it's conducting with the Food and Drug Administration (FDA) and state and local health officials. The agency reported that 11 of the affected people were hospitalized and no one has died; the cases happened between November 2023 and late March 2024.
None of the 22 people met the official case definition for botulism. This rare and potentially fatal illness occurs when botulinum toxin circulates in the blood and then damages the body's nerves. Botulinum toxin used in medical treatments and cosmetic procedures, known as Botox, causes botulism only very rarely, but it is possible.
Several of the sickened people were still given the antitoxin that treats botulism, just in case, because side effects from poorly administered Botox can resemble some of the early symptoms of the condition. Such symptoms can include blurred and double vision, constipation, incontinence, shortness of breath, weakness and difficulty lifting one's head.
Related: Infant's 'inconsolable,' day-long crying fits tied to botulism from honey
Initial reports of these botched Botox injections came out earlier in the month, with Tennessee and Illinois reporting cases of botulism-like illness. New York City later issued similar reports.
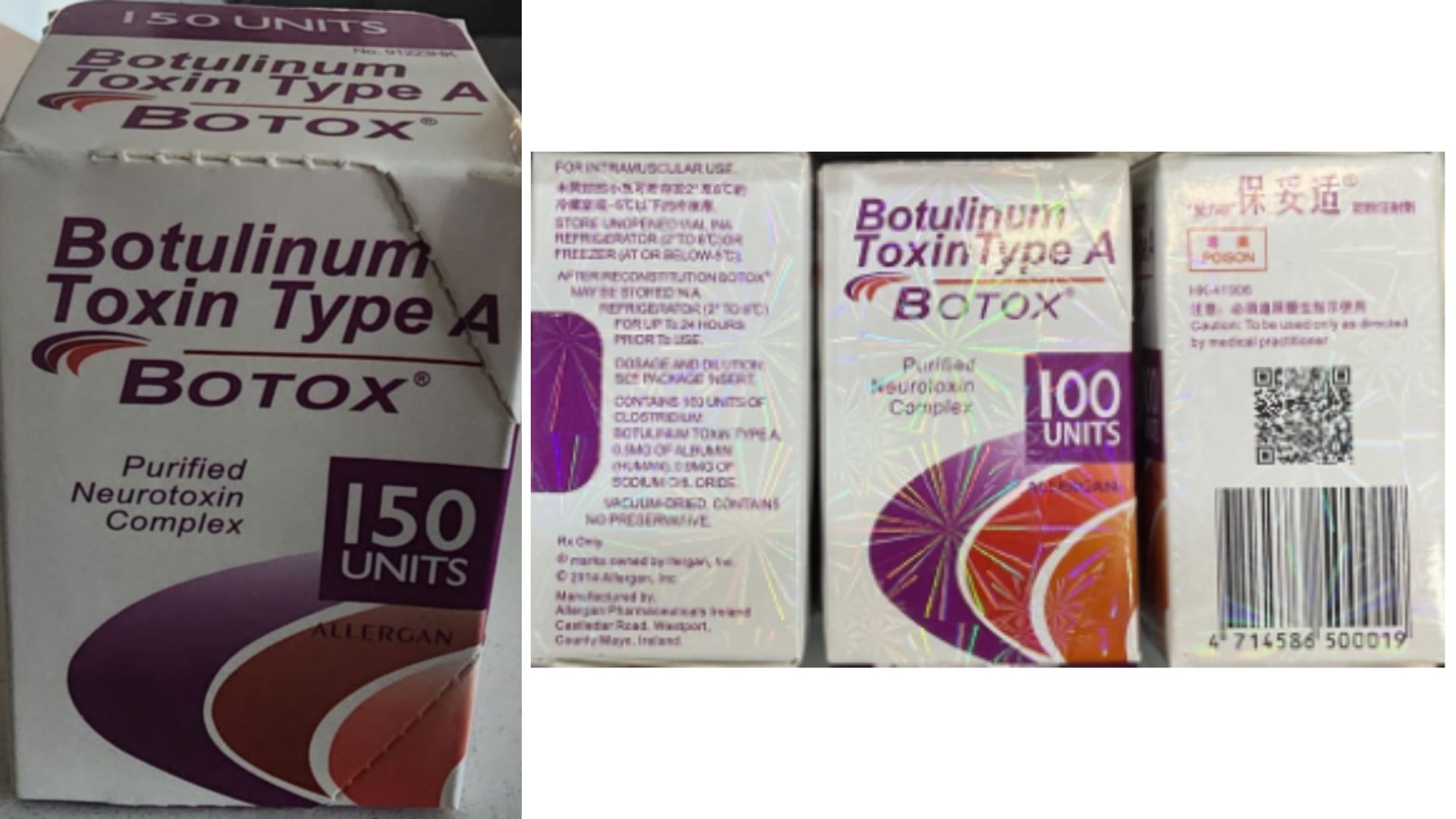
After investigating, the FDA issued a warning that "unsafe counterfeit versions of Botox" had been found in several states and were reportedly being used for cosmetic procedures. The agency included photos of some of the counterfeit products in its statement. "The products appear to have been purchased from unlicensed sources," the FDA noted.
The pharmaceutical company AbbVie makes FDA-approved Botox sold in the U.S., and the name of the company or its offshoot Allergan Aesthetics typically appears on the packaging. The FDA has found no evidence that AbbVie's approved Botox has been involved in the recent illnesses, so the genuine product is still safe to get from licensed professionals.
The FDA didn't note exactly what differed between the counterfeit products and the real deal, but said products from unlicensed sources may be "misbranded, adulterated, counterfeit, contaminated, improperly stored and transported [or] ineffective." Dr. Gregory Greco, the past president of the American Society of Plastic Surgeons, told NPR he suspects unlicensed providers are buying the fakes to cut costs and that these counterfeits may be highly concentrated, which would raise the risk of side effects.
As of April 23, illnesses related to the outbreak have been reported in California, Colorado, Florida, Illinois, Kentucky, Nebraska, New Jersey, Tennessee, Texas, Washington and New York. All of the people affected have been female and ranged from 25 to 59 years old. All reported getting the Botox injections from unlicensed or untrained individuals or in non-health-care settings, like spas.
Most people's symptoms emerged within three days of their injections, although some people started experiencing symptoms the same day or weeks later. Some symptoms affected the area immediately around the injection site — for instance, people who got facial injections experienced drooping eyelids and blurred vision. Other symptoms included dry mouth, slurred speech, shortness of breath, fatigue and generalized weakness.
The CDC recommends going to the emergency room immediately if you have any potential symptoms of botulism. If a doctor suspects a person may have the condition, they're advised to administer the antitoxin as soon as possible, without waiting on lab test results. Prompt treatment can prevent the toxin from doing more harm, especially to the nerves that support breathing.
The FDA also urges health care providers and consumers to report any side effects related to Botox injections to MedWatch, the FDA's safety information and adverse event reporting program. The agency's investigation into the counterfeit products is ongoing.
This article is for informational purposes only and is not meant to offer medical advice.
Ever wonder why some people build muscle more easily than others or why freckles come out in the sun? Send us your questions about how the human body works to community@livescience.com with the subject line "Health Desk Q," and you may see your question answered on the website!







