
A cruel consequence of advanced cancer is the profound apathy many patients experience as they lose interest in once-cherished activities. This symptom is part of a syndrome called cachexia, which affects about 80% of late-stage cancer patients, leading to severe muscle wasting and weight loss that leave patients bone thin despite adequate nutrition.
This loss of motivation doesn’t just deepen patients’ suffering, it isolates them from family and friends. Because patients struggle to engage with demanding therapies that require effort and persistence, it also strains families and complicates treatment.
Doctors typically assume that when late-stage cancer patients withdraw from life, it is an inevitable psychological response to physical deterioration. But what if apathy isn’t just a byproduct of physical decline but an integral part of the disease itself?
In our newly published research, my colleagues and I have discovered something remarkable: Cancer doesn’t simply waste the body – it hijacks a specific brain circuit that controls motivation. Our findings, published in the journal Science, challenge decades of assumptions and suggest it might be possible to restore what many cancer patients describe as most devastating to lose – their will to engage with life.
Untangling fatigue from physical decline
To unravel the puzzle of apathy in cancer cachexia, we needed to trace the exact path inflammation takes in the body and peer inside a living brain while the disease is progressing – something impossible in people. However, neuroscientists have advanced technologies that make this possible in mice.
Modern neuroscience equips us with a powerful arsenal of tools to probe how disease changes brain activity in mice. Scientists can map entire brains at the cellular level, track neural activity during behavior, and precisely switch neurons on or off. We used these neuroscience tools in a mouse model of cancer cachexia to study the effects of the disease on the brain and motivation.
We identified a small brain region called the area postrema that acts as the brain’s inflammation detector. As a tumor grows, it releases cytokines − molecules that trigger inflammation − into the bloodstream. The area postrema lacks the typical blood-brain barrier that keeps out toxins, pathogens and other molecules from the body, allowing it to directly sample circulating inflammatory signals.
When the area postrema detects a rise in inflammatory molecules, it triggers a neural cascade across multiple brain regions, ultimately suppressing dopamine release in the brain’s motivation center − the nucleus accumbens. While commonly misconstrued as a “pleasure chemical,” dopamine is actually associated with drive, or the willingness to put in effort to gain rewards: It tips the internal cost-benefit scale toward action.
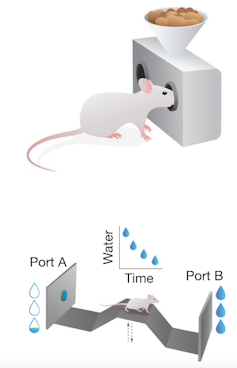
We directly observed this shift using two quantitative tests designed with behavioral economics principles to measure effort. In the first, mice repeatedly poked their noses into a food port, with progressively more pokes required to earn each food pellet. In the second task, mice repeatedly crossed a bridge between two water ports, each gradually depleting with use and forcing the mice to switch sides to replenish the supply, similar to picking berries until a bush is empty.
As cancer progressed, mice still pursued easy rewards but quickly abandoned tasks requiring greater effort. Meanwhile, we watched dopamine levels fall in real time, precisely mirroring the mice’s decreasing willingness to work for rewards.
Our findings suggest that cancer isn’t just generally “wearing out” the brain − it sends targeted inflammatory signals that the brain detects. The brain then responds by rapidly reducing dopamine levels to dial down motivation. This matches what patients describe: “Everything feels too hard.”
Restoring motivation in late-stage disease
Perhaps most exciting, we found several ways to restore motivation in mice suffering from cancer cachexia − even when the cancer itself continued progressing.
First, by genetically switching off the inflammation-sensing neurons in the area postrema, or by directly stimulating neurons to release dopamine, we were able to restore normal motivation in mice.
Second, we found that giving mice a drug that blocks a particular cytokine − working similarly to existing FDA-approved arthritis treatments − also proved effective. While the drug did not reverse physical wasting, it restored the mice’s willingness to work for rewards.
While these results are based on mouse models, they suggest a treatment possibility for people: Targeting this specific inflammation-dopamine circuit could improve quality of life for cancer patients, even when the disease remains incurable.
The boundary between physical and psychological symptoms is an artificially drawn line. Cancer ignores this division, using inflammation to commandeer the very circuits that drive a patient’s will to act. But our findings suggest these messages can be intercepted and the circuits restored.

Rethinking apathy in disease
Our discovery has implications far beyond cancer. The inflammatory molecule driving loss of motivation in cancer is also involved in numerous other conditions − from autoimmune disorders such as rheumatoid arthritis to chronic infections and depression. This same brain circuit might explain the debilitating apathy that millions of people suffering from various chronic diseases experience.
Apathy triggered by inflammation may have originally evolved as a protective mechanism. When early humans faced acute infections, dialing down motivation made sense − it conserved energy and directed resources toward recovery. But what once helped people survive short-term illnesses turns harmful when inflammation persists chronically, as it does in cancer and other diseases. Rather than aiding survival, prolonged apathy deepens suffering, worsening health outcomes and quality of life.
While translating these findings into therapies for people requires more research, our discovery reveals a promising target for treatment. By intercepting inflammatory signals or modulating brain circuits, researchers may be able to restore a patient’s drive. For patients and families watching motivation slip away, that possibility offers something powerful: hope that even as disease progresses, the essence of who we are might be reclaimed.
Adam Kepecs receives funding from the National Institutes of Health.
This article was originally published on The Conversation. Read the original article.







