Throughout human history, mosquitoes have constantly buzzed in the background of human existence, irritating us with their incessant bites and occasionally wreaking havoc by transmitting deadly diseases. The earliest known mosquitoes from the fossil record date back at least 70 million years, and evidence of mosquito-borne diseases like malaria dates back to Egyptian mummies from 2000 BC.
Apart from malaria, which claims the lives of over half a million people every year and infects close to 250 million, mosquitoes serve as vectors for various other diseases. These include dengue, Zika, lymphatic filariasis, and yellow fever. Understandably, our relationship with these tiny, blood-sucking insects has been far from cordial.
Help from sequencing tech
The rapid urbanisation of the world’s populations, especially in many large and economically developing countries like India, has led to annual surges in mosquito-borne illnesses like dengue. Together with climate change and its cascading consequences, mosquito-borne diseases have expanded into new territories. A notable example is the indigenous cases of dengue in France in recent years.
It is not surprising then that mosquito control has taken centrestage today and the battle continues unrelenting with an array of tools – from mosquito nets to insecticides and the use of symbionts like Wolbachia. But with insecticide resistance in mosquitoes rising to alarming proportions, it has become imperative that newer approaches to mosquito control gain prominence, even despite the availability of a first-generation malaria vaccine that officials in different countries are currently implementing in a pilot in endemic regions.
In the last two decades, our ability to read or sequence the genomes of organisms, and edit and manipulate these genomes, has given us new tools in this fight. Thanks to advancements in next-generation sequencing techniques, we now have access to the whole genome sequences of multiple mosquito species. Notably, researchers from the University of California, the Tata Institute of Genetics and Society, and the Institute of Bioinformatics and Applied Biotechnology have helped prepare high-quality reference genomes for Anopheles stephensi, a major malaria-vector mosquito. (The two latter institutes are based in Bengaluru.)
The availability of these high-quality sequences, coupled with our capacity to genetically manipulate them, offers unprecedented opportunities.
The gene drive
The fundamental idea behind genetic manipulation of mosquitoes is to systematically control their populations by interfering with their reproduction. Scientists worldwide have developed various genetic modification approaches. A major one in this endeavour is gene-drive technology, whose end result is for mosquitoes to selectively inherit some genes, rather than the inheritance to follow the rules of Mendelian genetics.
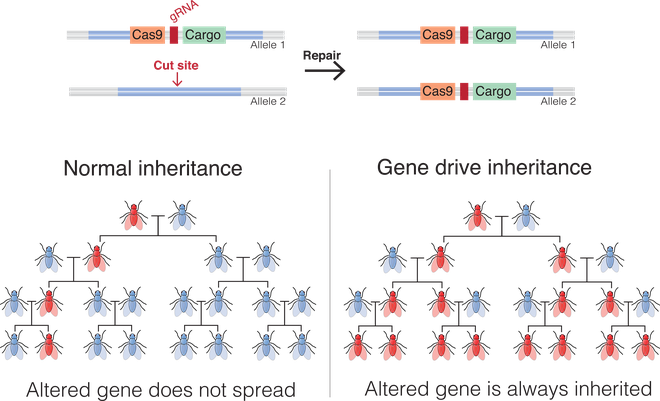
This technology was conceived by Austin Burt, professor at Imperial College London, in a 2003 paper published in Royal Society Proceedings. Here, a protein cuts the mosquito’s DNA at a part that doesn’t encode a particular sequence in the genome. This triggers a natural mechanism in the cell containing the DNA to repair it and forces the cell to incorporate a sequence, called the drive sequence, into the damaged portion.
Numerous gene-drive versions are documented in the literature, but they all focus on reducing mosquito populations’ reproductive capabilities or rendering them sterile. As a result, the malaria parasite won’t be able to replicate inside the mosquito’s gut. In a recent paper in Science Advances, researchers at Imperial College London genetically enhanced a gene expressed in the midgut of mosquitoes to secrete two antimicrobial substances called magainin 2 and melittin. They are detrimental to the Plasmodium parasite’s development in the midgut and also reduce the lifespan of female mosquitoes. Computational modelling studies have suggested that this approach could significantly disrupt malaria transmission.
Benefits as well as risks
In 2020, the U.S. Environmental Protection Agency authorised the release of a genetically modified mosquito called OX5034 in counties in Florida and Texas. Oxitec developed this mosquito with a gene sensitive to an antibiotic, tetracycline. The authorisation followed extensive field trials conducted by the company in Brazil. Genetically modified male OX5034 mosquitoes mated with female mosquitoes but the self-limiting gene prevented female offspring from surviving. As a result, the male mosquitoes would disappear from the environment after around a dozen generations.
Genetically modified mosquitoes have been used in outdoor but controlled conditions in India, Brazil, and Panama as well. Early results from these trials showed promising drops in mosquito populations, around 90% during the trials. Additional trials have shown that such technologies can decrease incidence of dengue significantly as well.
These technologies can bring benefits as well as risks, in different ways. An immediate implication is that the drastic reduction in the mosquito population could alter food chains and ecosystems that involve mosquitoes. So it’s likely that the gap in the food chain could be ‘invaded’ by other mosquitoes or in fact other insects. As such, we don’t fully understand the implications in the short and longer terms. And since the consequences will be shared by individuals, communities, and populations, in that order, what constitutes a right decision and what processes are to be followed remains a dilemma to policymakers.
A timeless struggle
It is therefore not surprising that the genetic engineering of mosquitoes and trials involving genetically modified mosquitoes have faced multifaceted challenges in different parts of the world. Critics have expressed concerns about unintended consequences, such as unforeseen ecological disruptions or the potential for engineered genes to spread beyond target mosquito populations.
Some of these concerns are valid and require extensive data collection, close monitoring, and multistakeholder discussions surrounding the adoption of this technology. Closer home, on the regulatory front, the Department of Biotechnology released comprehensive guidelines for genetically engineered insects earlier this year. They provide a roadmap for researchers, outlining procedures and regulations for working with such insects in the country.
The battle between mosquitoes and humankind seems to be a timeless struggle, a testament to human ingenuity against frustrating troublemakers aided by evolution.
The authors are senior consultants at the Vishwanath Cancer Care Foundation.







