Nearly half the adults in the U.S. have high blood pressure. This adds up to around 108 million people, according to the Centers for Disease Control and Prevention.
High blood pressure contributed to nearly 500,000 deaths, the CDC says, and only one in four people have their condition under control.
The agency said high blood pressure costs the nation about $131 billion to $198 billion each year. The total includes the cost of health-care services, medications to treat high blood pressure, and loss of productivity from premature death.
"Fortunately, high blood pressure is preventable and treatable," the CDC said.
Quinapril is a treatment for hypertension that was patented in 1980 and came into medical use in 1989.
It is available as a generic medication. In 2020, it was the 253rd most commonly prescribed medication in the U.S., with more than 1 million prescriptions.
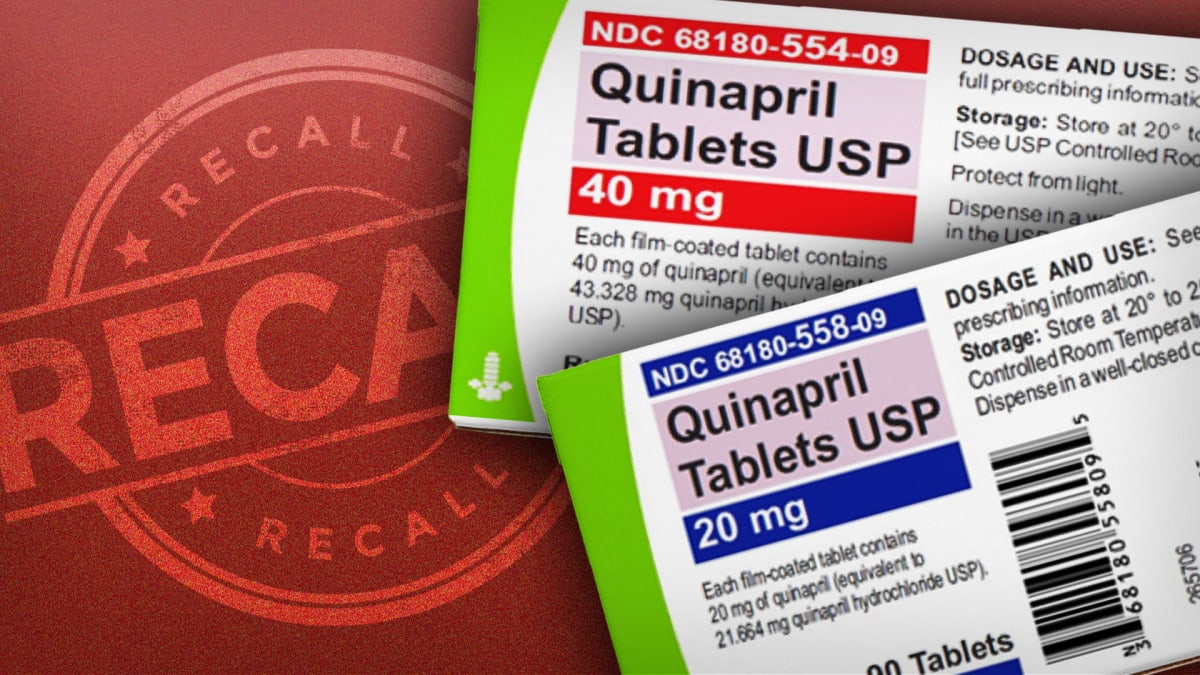
Quinapril was the subject of a recent recall, according to the U.S. Food and Drug Administration.
The FDA says Lupin Pharmaceuticals of Mumbai voluntarily recalled four lots of the medication, saying it had too much of an impurity called N-Nitroso-Quinapril.
To date, Lupin, which is the second company to have recalled Quinapril recently, has received no reports of illness that appear to relate to this issue, the FDA said on Dec. 21.
Nitrosamines are common in water and foods, including cured and grilled meats, dairy products and vegetables.
"Everyone is exposed to some level of nitrosamines," the FDA said in a statement. "These impurities may increase the risk of cancer if people are exposed to them above acceptable levels over long periods of time."
Second Company to Recall Quinapril
The affected lots were distributed from March 2021 to September 2022 and have expiration dates ranging from December 2022 to March 2024. They came in 90-count bottles. The affected lot numbers are listed on the FDA website.
Lupin Pharmaceuticals is notifying its wholesalers, distributors, drug chains, mail order pharmacies and supermarkets by phone and through recall notification and is arranging for the return of all the recalled product lots.
Patients taking Quinapril Tablets USP 20mg and 40mg are advised to continue taking their medication and contact their pharmacists, physicians, and medical providers for advice regarding an alternative treatment.
Wholesalers, distributors and retailers that have Quinapril Tablets USP 20mg and 40mg that are being recalled should stop distributing the recalled product lots immediately.
Lupin Pharmaceuticals said it stopped making the drug in September and was working with distributors to arrange for the return of the recalled product lots.
In 2021, Lupin voluntarily recalled batches of the blood pressure treatment Irbesartan tablets and Irbesartan and Hydrochlorothiazide tablets.
Lupin is the second company to recall Quinapril.
Company Subject to FDA Inspections
Aurobindo Pharma recalled two lots of Quinapril in October, noting the same nitrosamine impurity.
Based in East Windsor, N.J., Aurobindo Pharma USA is a subsidiary of the Indian pharmaceutical company Aurobindo Pharma Ltd.
Aurobindo Pharma Ltd. has recalled several products this year including moxifloxacin ophthalmic solution — an antibiotic used to treat eye infections like bacterial conjunctivitis, according to Endpoints News.
In January, Aurobindo voluntarily recalled a lot of polymyxin B for injection after a hair was found in a vial within the lot.
The company, which has been subject to several FDA inspections, earlier this year shut down its site in Dayton, N.J., eliminating 99 jobs.
Aurobindo said it had received a so-called 483 inspection report from the FDA regarding the company’s oral manufacturing facility in Hyderabad, India. The agency made six observations, which were not detailed.
A Form 483 is issued when investigators at the FDA observe conditions that in their judgement “may constitute violations” of the Food Drug and Cosmetic Act and related laws and regulations.
Also in October, Golden State Medical Supply recalled bottles of Atenolol, a blood pressure medication, and Clopidogrel, a medication aimed at heart issues, after the manufacturer discovered that labels had been switched on some of the bottles.
Give Yourself the Gift of Future Returns!
You’re invited to join Action Alerts PLUS for just $79.99/yr. Don’t miss this chance to unlock best-in-class investing guidance from trusted portfolio managers -- without the management fees.







