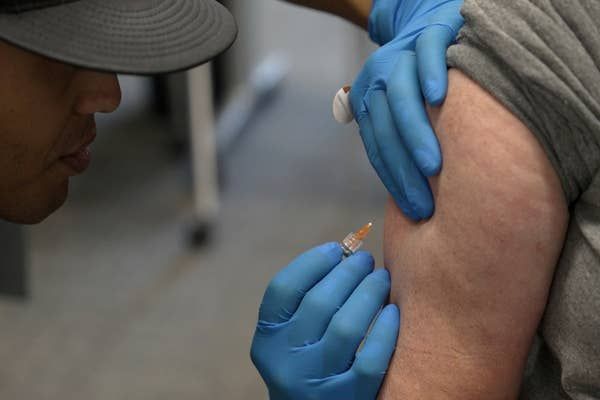
The arrival of GLP-1 analogue drugs (such as Wegovy) marked a huge shift in the weight loss drug market. These drugs have been shown to lead to significant weight loss in users – as much as 15% or more of their body weight in clinical trials. For this reason, demand for weight loss drugs has skyrocketed worldwide.
Most of the GLP-1 analogue drugs on the market are taken as a weekly injection under the skin. But many companies are now working on translating these drugs into a form that can be taken orally, as a pill.
But will weight loss pills be as effective as the injectable GLP-1 drugs already on the market?
How do injectable weight loss drugs work?
When we eat, the gastrointestinal system produces a variety of hormones in response, that go on to signal satiety to the brain. Collectively, these hormones are called “incretins”. Glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP) and islet amyloid polypeptide (amylin) are all incretins.
Incretins signal the hypothalamus (a structure in the brain that links the endocrine and nervous systems) and other brain regions to tell the rest of the body we’re full.
The drug semaglutide (sold under the brand names Wegovy and Ozempic) mimics the naturally occurring incretin GLP-1. But unlike the GLP-1 the body produces (which is quickly broken down by enzymes after it’s been released), semaglutide has been pharmacologically modified so that the hormone lasts longer in the body – thereby making a person feel fuller for longer after meals.
Other weight loss drugs can act on more than one incretin receptor. Tirzepatide (sold under the brand name Mounjaro) is the first available “dual” incretin. In other words, it has properties of two incretin molecules – acting on both GLP-1 and GIP receptors. Clinical trials showed tirzepatide is even more effective than semaglutide – leading to an impressive loss of up to 20% of body weight in overweight or obese participants who took the drug for 72 weeks.
How would a weight loss pill work?
Novo Nordisk, the makers of Wegovy, recently announced the phase 1 trial results of a new oral weight loss pill they’re developing, called amycretin.
At the European Association for the Study of Diabetes conference in September 2024, researchers reported that in early trials, amycretin led to a 10.4% loss of body weight in people who were overweight or obese when taken at its lowest dose for 12 weeks. When taken at a higher dose, it led to an over 13% loss of body weight in the same time period. This was compared to participants who were given a placebo, who lost only 1% of their body-weight. The amount of weight lost was faster than when compared to semaglutide injections.
Amycretin works by targeting two incretin hormone receptors: GLP-1 and amylin.
Amylin is secreted at the same time as insulin by cells in the pancreas. The hormone plays a key role in blood sugar (glucose) regulation by controlling how quickly food is digested in the stomach and controlling when the hormone glucagon in released. Importantly, amylin receptors are found in specific brain regions that regulate appetite. As such, it can trigger a satiety signal after meals.
Weight loss from GLP-1 and amylin receptor treatments works through both separate as well as shared brain pathways. Because of this, combining the two therapies is anticipated to have a greater effect on promoting weight loss. Based on the early results from this amycretin trial, it appears this is true.
As amycretin has been developed to be taken as a pill, it may offer a more convenient option for managing obesity. Furthermore, weight loss had not plateaued by the 12-week period – so people may be able to lose more weight than that shown so far. And, side-effects appear similar to other incretin-based treatments – including nausea, vomiting, diarrhoea or constipation.
Larger trials are now taking place to better understand how safe and effective it really is.
Advances in the field of weight loss drugs are evolving rapidly. Further dual-agonist and even triple-agonist incretin drugs are in development. Trials so far show they lead to a significant loss of body weight. Numerous companies are also working on developing weight loss pills that target incretin receptors – with trial results for some anticipated later this year.
Amycretin has now advanced to the next phase of clinical trials. If proven to be as safe and effective as it was in phase one trials, it could be good news for patients with obesity. More options available on the market would also be good news as it could help manage global demand for weight loss drugs and ease the resultant shortages of these products.
Martin Whyte has received research funding from AstraZeneca.
This article was originally published on The Conversation. Read the original article.