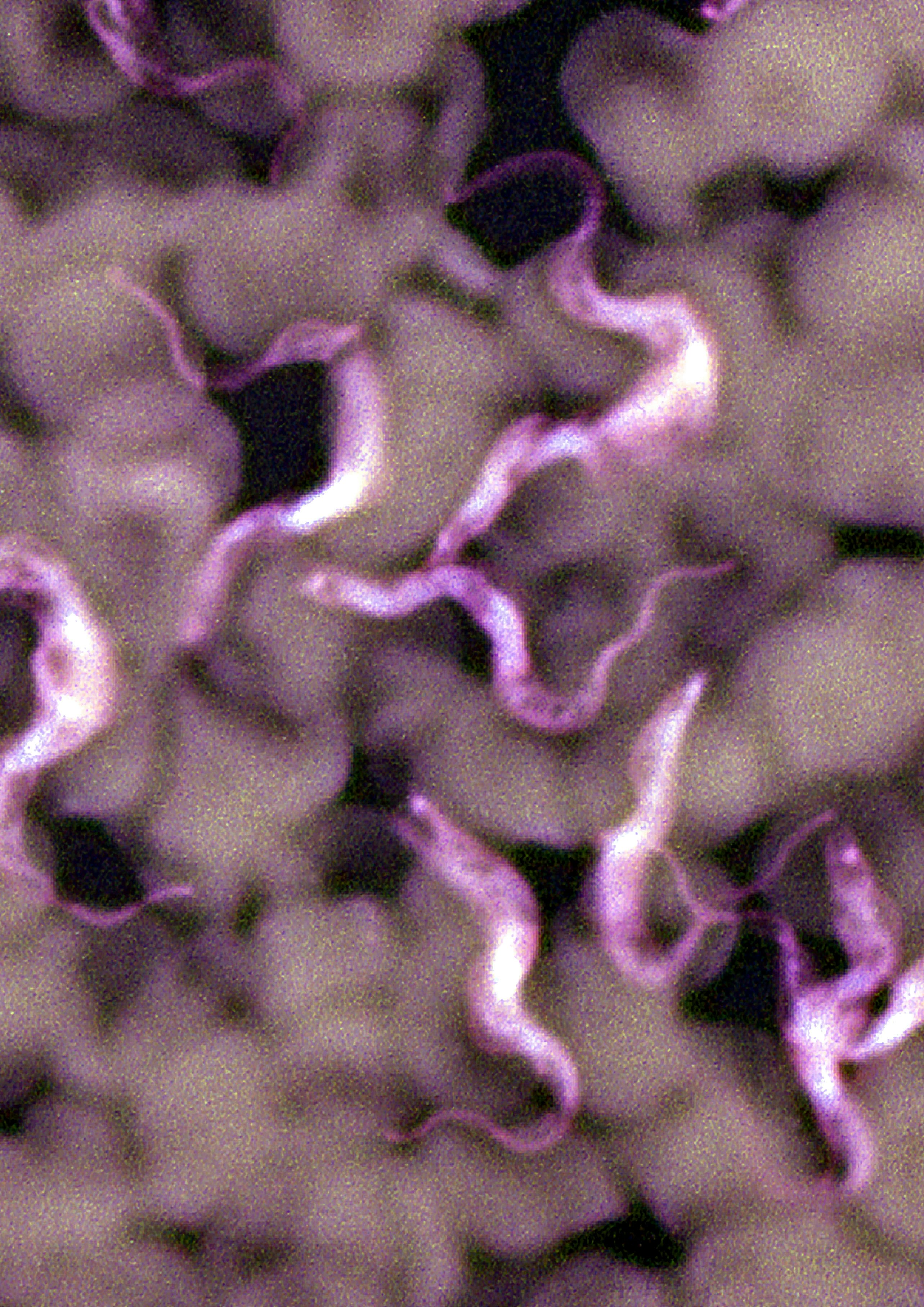
For people who live in 36 sub-Saharan African countries, one bite from the endemic tsetse fly can spell death. These creatures — and the parasites they may carry — have driven at least three epidemics in the last 120 years in this region, putting millions of lives at risk.
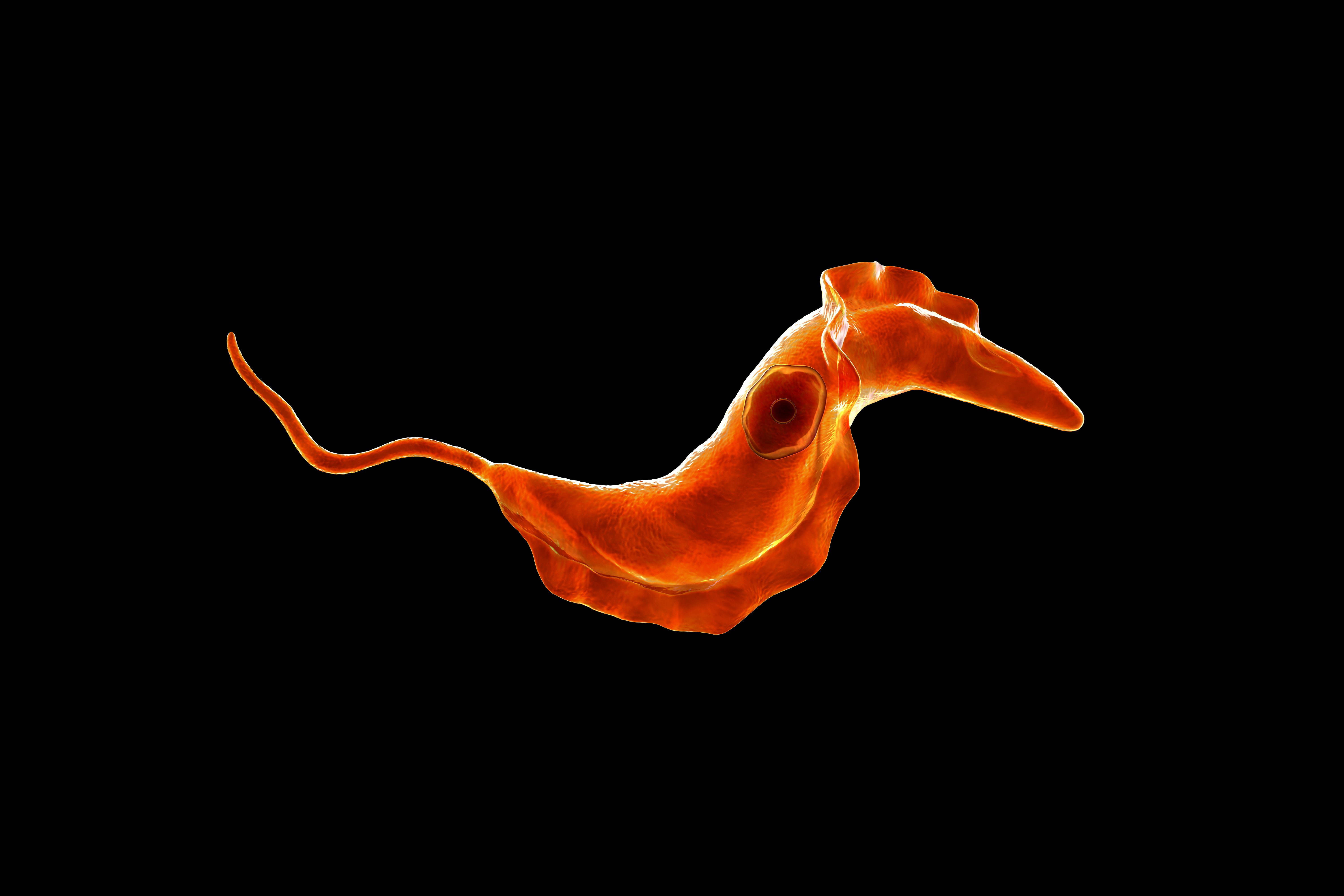
The reason why people fear the tsetse fly’s bite is that it can come loaded with a parasite, Trypanosoma brucei, which infects the blood and nervous system and causes the disease African sleeping sickness.
The disease is treatable, but only if you have access to the special medication, doctors, and other care you need to survive — if left untreated, African sleeping sickness is almost always fatal. Now, an international research team believes they have a new way to curb the parasite — one that relies on the parasite’s own genetic blueprint.
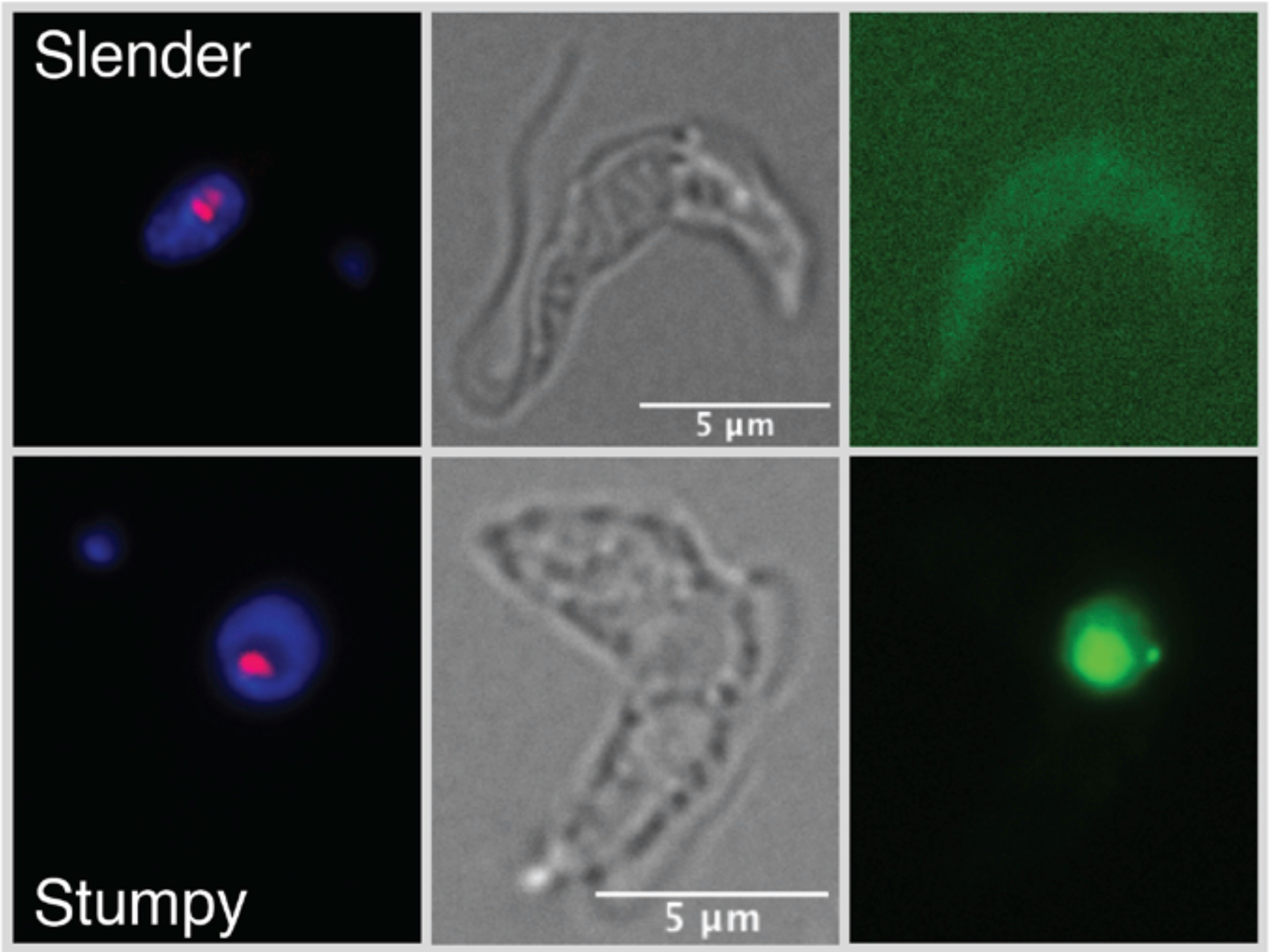
What’s new — In a paper published Wednesday, June 15 in Science Advances, the team describes how they discovered a gene that transforms the slender, worm-like parasite into a short, stumpy form — the moment in the parasite’s lifecycle when it stops reproducing and dies. After a person is bitten by a tsetse fly carrying the parasite, the parasite infects the blood and begins reproducing until it can no longer sustain its growth. At that point, it morphs.
The gene that triggers this physiological change, which the researchers named grumpy, produces RNA — a kind of genetic messenger — that could speed up this metamorphosis. Previously, researchers had only been able to slow the infection down, study co-author Luísa Figueiredo tells Inverse. Figueiredo leads a lab devoted to study parasites at the University of Lisbon.
African sleeping sickness is a neglected tropical disease, meaning it generally affects the people with access to the fewest resources in rural areas of the world. In turn, it receives relatively little attention from the global public health community.
The early phases of infection are difficult to spot, so most people are only diagnosed once the parasite makes it into their brain. At that point, a person may exhibit behavior changes, confusion, and the sleep disturbances that inspire the disease’s name, according to the World Health Organization.
The new findings could help improve the diagnosis of the disease and to develop new treatments for African sleeping sickness using genetic interventions, according to study co-author Fabien Guegan. Guegan is a senior postdoctoral researcher at the University of Lisbon.
Why it matters — African sleeping sickness is deadly if left unchecked, but it is treatable — if you can get it, that is. Current treatments for the disease are complex and have significant side effects says Marina Antillon, who is a scientist at the Swiss Tropical and Public Health Institute. Antillon was not involved in the new study.
Trypanosoma brucei plays the long game. Once the parasites have produced enough copies of themselves, some will begin morphing from their usual slender form into a shorter, stumpy version that cannot reproduce. From there, the stumpy cell will either be taken back up by another blood-sucking fly or die within four days, limiting the parasite’s population. This allows the host to stay alive, which is why the infections can last years.
As the early stages of the disease have few visible symptoms, diagnosing an infection before the parasite unleashes real damage on the host’s nervous system is a challenge — especially after the parasite makes it into the brain tissue.
But if we can better understand the parasite’s genome, it means scientists can look for genetic clues in a host’s blood or urine to detect the parasite earlier in the infection — and even develop gene-targeting treatments to halt its progress.
How they did it — In the new study, scientists identified one gene that encourages the cells to morph into their stumpy form earlier than would be typical. In large-enough amounts, the RNA that this gene produces lowers the number of parasites in the blood of infected mice, according to the study.
The scientists named the gene grumpy, a portmanteau of “growth” and “stumpy.”
“The pathways regulating this switch have been of great interest in the field for a long time,” Matthias Marti, professor of molecular parasitology at the University of Glasgow who was not involved in the research, tells Inverse.
“The study is a real tour de force.”
The researchers discovered grumpy by combing through a part of the genome that scientists once dubbed “junk DNA,” which is basically non-coding DNA. DNA is essentially a set of biological instructions for building proteins, the complex strings of amino acids that allow your body to function. But protein-coding genes account for as little as one percent of the genome — these are known as the exome. The remaining 99 percent of the genome is made of noncoding genes.
Noncoding does not mean not important, however, but scientists are still unpacking the many roles these genes may play. One variety, long noncoding RNA genes, sometimes get involved in altering a cell’s shape and function — like precipitating a shift from an active, slender form to a short, stumpy form.
Knowing this, the researchers analyzed the RNA produced from the parasite’s genome and found 1,428 previously unidentified long noncoding RNA genes, 399 of which may play a role in the parasite’s transformation. They narrowed in on grumpy because of its location next to a few coding genes known to be involved in the process. When they artificially increased grumpy’s RNA product, most of the parasites in infected mice morphed into their stumpy form.
What's next — This research is preliminary, but now researchers have characterized grumpy and seen how upping its activity can quell an infection in mice, they can look toward translating the findings to humans. Looking for parasitic RNA in a person’s blood and urine may diagnose an infection before it gets to the brain — but the gene’s function may be a potential vulnerability that can be exploited to treat the disease, too.
A direct treatment that neutralizes the parasite by triggering a premature shift to its stumpy form would be highly effective, according to some parasite experts.
“Accelerating the process has the beneficial potential to reduce parasite virulence or limit spread,” Keith Matthews, who studies the parasite at the University of Edinburgh and was not involved in the research, explains to Inverse. Such a treatment is still years away from fruition, but the finding is an indication of the potential for gene targeting to quash parasitic infections.
Ultimately, grumpy is only one of more than a thousand similar T. brucei genes, including hundreds that may affect the parasite’s transformation to its stumpy form. These findings have been uploaded to a database that will allow scientists around the world to investigate the role that these genes play in the parasite’s life cycle.
Next, the researchers will examine how levels of different long noncoding RNAs change as the disease progresses, Guegan says. Hopefully, this information can be used to determine the best marker of the disease for future diagnostic tests.







