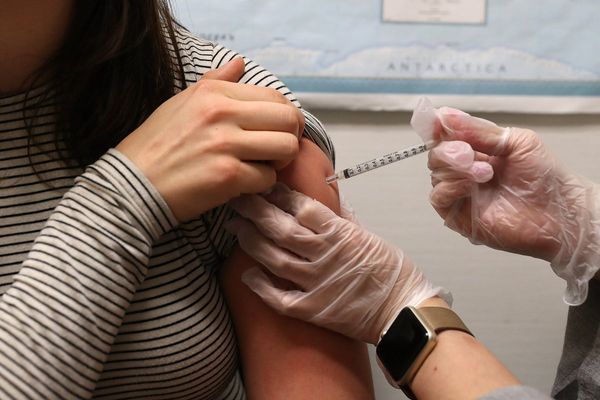
As she prepared her medical-device startup for a make-or-break clinical trial, one of Anne Morrissey’s first thoughts was, There’s no way we can do this clinical trial.
There were the usual challenges that test any health care startup, including financial worries—did they have enough money to hire a team, train staff, compensate investigators, gather sufficient data? But there were also some uniquely convoluted ones. Like: When and how could they get informed consent from patients on the verge of giving birth?
It was 2016, and Morrissey was the new CEO of Alydia Health, a five-year-old company with a novel device, known as the Jada System, to treat postpartum hemorrhage (PPH), or excessive bleeding after delivery. PPH is a potentially devastating complication of childbirth that affects 14 million women a year, including hundreds of thousands in the U.S. And while deaths from PPH are largely preventable—and rare, in rich nations—it still kills 70,000 women every year globally, ranking as the leading cause of maternal death worldwide.
As medical devices go, Jada, a wand-like tool that is inserted in the uterus, was unbelievably simple. But as a device designed to address a pregnancy-related problem—an area of medicine rarely touched by innovation—its path to approval was anything but straightforward.
PPH is an emergency complication—often sudden and hard to anticipate. A woman can’t volunteer for a trial while she’s rapidly losing blood and in need of lifesaving care. Nor it seemed, could she do so while in active labor, when she may be under duress. (FDA rules require that patients be given sufficient opportunity to consider participation in a trial, in a period of clear thinking where they’re free of coercion.) But it wouldn’t work to “consent” women at prenatal appointments, either, due to the paperwork nightmare of getting the relevant forms from doctors' offices to hospitals.
By 2017, Alydia, the FDA, and the clinical trial sites had finally worked out an elaborate protocol around informed consent: allowing the process during the first stage of labor, but not the second, or while contractions or medical interventions were underway.

That matter settled, Morrissey’s team moved onto the next doozy: how to sensitively discuss the benefits of participating in an experimental study—and raise the possibility of additional risks—with women who were about to undergo the physically intense and profoundly vulnerable experience of childbirth. And how many would you have to ask, given that only a fraction of them would suffer hemorrhage?
In the end, more than 7,000 women provided consent to reach the 107 U.S. PPH cases Alydia needed to power its study. “It was really slow,” remembers Morrissey—so slow that, in the beginning, the team celebrated each additional case by opening a bottle of champagne. But as word of success spread at trial sites, the pace of enrolling patients picked up.
In 2020, five painstaking years after the trial was first being planned, that success began to bear fruit. Data published in Obstetrics & Gynecology, a preeminent journal, showed Jada was effective in 94% of the hemorrhage cases, controlling bleeding in a median time of just three minutes. That same year, Jada received FDA clearance. And in March 2021, Alydia sold for $240 million to Organon, a women’s health-focused company that was being spun out of Merck, the giant American drugmaker.
It was an eye-popping exit for Alydia, a crowning and previously almost unthinkable achievement for a team of people who willed the Jada, on a shoestring budget, through 10 twisty, turny years of development. The founders hadn’t doubted the technology, only that they’d ever get through the obstacle-laden path to bring it into the world.
The odds are long for most medical innovations, but especially so in women’s health, a field that remains underdeveloped—trapped by old-fashioned gender dynamics that still stubbornly guide the market today.

Alydia was selling a product that had a potential market of millions of women a year, one that could save lives and money too. But up until Alydia’s acquisition, that was a value proposition that almost no one wanted to bet on. As a women’s health product—and not, say, a cancer drug—the Jada was an opportunity that simply didn’t compute in most investor models and mindsets.
That left Alydia, like a lot of companies in its neglected field, under-resourced for a development process that in nearly every aspect is tougher for women’s health companies: the science less clear, the regulators more demanding, the history more fraught. Even the hard-won success stories are modest, flotsam and jetsam in a multitrillion-dollar industry’s deal flow. The fate of Jada is a case in point: It’s now tied to the future of Organon, an ambitious but unproven company that’s arguably even more at the mercy of financial markets that haven’t shown much faith in women’s health.
There are signs—and certainly hopes—that such neglect and uninterest are giving way to something more equitable. And that makes the story of Alydia and Organon a parable of the women’s health business, emblematic of the challenges that have long thwarted innovation and the forces that could change the industry for the better.
Investing in women, in a system that doesn’t
“Here for Her Health,” proclaimed the banner on the façade of the New York Stock Exchange on the rainy morning in June 2021 that Organon made its public-market debut. The company’s arrival seemed well timed to capitalize on the cultural moment and the energies of #MeToo and FemTech—the name given to the women’s health startup industry.
More than a splash of marketing savvy went into the ceremony—Organon executives rang the opening bell and posed for photographs under fuchsia umbrellas. But the company’s launch also represented something bold and unusual, a fist raised to the male-centered medical establishment, in the form of a business with a $9.4 billion market cap that was proudly, explicitly investing in women’s health.
Organon’s ambitions for innovation are expansive, ranging from diagnostics and devices to drugs and digital therapeutics for conditions exclusive to women, like endometriosis, and to those that disproportionately affect them, like migraine or overactive bladder syndrome. The company has moved quickly, doing six deals in its first 16 months, a spree that included Alydia, a Finnish biopharma company, and an agreement to develop an on-demand, non-hormonal contraceptive.

Sandy Milligan, the company’s R&D chief, has a lot more on her wish list. When Fortune spoke with her this fall, her team had already looked at 220 companies. She rattled through priorities—a diagnostic for preterm labor, treatment for preeclampsia, digital therapeutics for menopause—giving the sense that both the possibilities and needs are endless. “It’s blue sky,” says Milligan.
It’s a puzzle of the $4.3 trillion American health care market that investing in women’s health should be either novel or bold. After all, women account for more than half the population, and 80% of all health care purchasing decisions. They take more prescription drugs and more often go to the doctor. But despite their market power, for much of history, women—their perspectives and biology—have largely been an afterthought or inconvenience for the broader industry.
Medical education, diagnostics, devices, drugs—even some drugs intended for women—were largely developed using men and an understanding of male biology. It wasn’t until 1993 that the government required women to be included in the health studies it helped fund, and not until 2016 that it required the same of cells and animals used in research.
Nor have their needs been the focus of much innovation. In 2020, just 1% of all biopharma R&D spending went towards female-specific conditions, excluding oncology according to McKinsey. (Adding oncology, you get to 5%.) In the medical-device industry, it was 4%. The FemTech movement, which saw funding increase from $499 million in 2018 to $1.5 billion in 2021, still amounts to only 5% of digital health investment in the U.S., according to venture fund Rock Health. Large corporate actors, meanwhile, have fled: “A lot of companies move away, as it’s proven challenging to innovate,” says Jason Gerberry, a Bank of America analyst who covers Organon.
Challenging, and sometimes downright dangerous. The history of the women’s health business is littered with misguided products—like thalidomide, the anti-nausea drug for pregnant women that wound up causing birth defects, or the Dalkon Shield IUD, which was linked to problems like pelvic infection and infertility—and companies hobbled by litigation over them.
The costs of this longstanding bias are evident in statistics that show how poorly women have been served by centuries of medical development. Women suffer adverse drug reactions nearly twice as often as men. They must wait longer and work harder to get a diagnosis, and they are more often misdiagnosed or likely to have legitimate concerns—like a heart attack—dismissed. There’s a whole category of health issues that have been ignored, viewed as the unfortunate discomforts of aging or pregnancy, rather than as needs that could be met. And for conditions that haven’t been ignored, the solutions are often crude. “I can’t tell you the number of times I’ve heard male physicians say, ‘Oh, we’ll just take her ovaries out,’” says Surbhi Sarna, who founded a company focused on better detection of ovarian cancer.
The unjustness of this status quo has been a subject of advocacy for decades, but change has been halting and slow. Businesswoman Carolee Lee—she founded accessory company Carolee Designs—has long been an activist on this front. In 2020, her organization, Women’s Health Access Matters (WHAM) commissioned a study from RAND on the value of women’s health research. The firm found that investing just $300 million—a pittance by health care standards—would reap a $13 billion return on investment in terms of “quality of life, reduced healthcare costs and more productive years added back to our workforce.”
Lee’s instinct to focus on dollars is a smart one, as economic assumptions are perhaps the biggest barriers to innovation. “It’s believed you can’t make money in women’s health,” says Dr. Elizabeth Garner, chief scientific officer for Ferring, a privately held, 72-year-old Swiss company that's a leader in the field. Garner and others describe that self-reinforcing notion as a brick wall that entrepreneurs and innovators consistently crash into. Valuations of women’s health companies are depressed because there are few success stories to point to, and there are few success stories because they struggle to get sufficient investor support. And with male investors and executives still far more likely than female ones to be calling the shots, women’s health has been that much more likely to wind up in the industry’s discard pile.
This is the narrative that Organon is trying to change. At the company’s May 2021 Investor’s Day, Organon CEO Kevin Ali (yes, he’s a man, though 40% of Organon’s executive leadership team and 70% of its board are women) made the case for betting big on women’s health. He led investors through a data-packed spiel on the staggering market sizes, the wide-open playing field, the bounty of affordable valuations of acquisition targets, the spending power of women. In all, he described it as a $61 billion opportunity by 2026.
The analysts at the virtual event, almost all of them male, weren’t particularly curious about these ambitions. One, Steve Scala of Cowen, seemed bewildered and somewhat antagonistic towards them: “I would like to go back to the most basic of points and ask why it makes sense to focus predominantly on women's health?” He pressed Ali on why a focus on cardiology or respiratory medicines wouldn’t be better. Like a polished executive, Ali said “great question” and went back to his talking points, with more feeling. (Scala declined to comment for this story.)
A year and a half later, the market has yet to match Ali’s enthusiasm. Organon’s current share price is 15% off its June 2021 debut; that compares to -7% for the S&P 500 and an 11% return for an index of health care companies over the same period. In October, CNBC pundit Jim Cramer weighed in on the stock. “It’s been a real dog…no thank you,” he exclaimed, though he noted that it wasn’t obvious why that was the case. (The company notes that about 80% of its revenue is from outside the U.S., so a strong dollar has impacted its top-line numbers.)
When Ali spoke to Fortune this fall, he expressed a certain impatience with the investment community—“it’s pretty much male-dominated,” he noted—and its failure to grasp what he does. “I think the market is a lagging indicator of what future performance is going to look like,” he said, adding that Organon has delivered on its earnings expectations quarter after quarter. Ali had grown resigned to the idea that, in terms of the promise of women’s health innovations, investors were going to have to see it to believe in it.
From student project to startup
Lack of imagination is a consistent theme in the women’s health field. Take the Jada. When asked about the device, obstetricians-gynecologists have a common response: “Why didn’t I think of that?” They often describe the tool as “elegant” and “basic.” Why it took until 2011 for someone to invent such a useful and obvious technology, they couldn’t tell me.
All the more fascinating, then, is how the work on Jada began—not with an entrepreneurial physician or in a medical-device company’s R&D division, but as an undergraduate student project.
The assignment, put to Alex Norred and his biomedical engineering classmates at California Polytechnic State University, had been to develop “a better balloon tamponade.” The goal was a noble one: The balloon tamponade had been an important tool for treating PPH since the late ‘90s, but marketed versions of the device were far too expensive for the low-resource health systems that most needed them.
Most often, when a woman experiences postpartum hemorrhage it’s because her uterus hasn’t contracted well after giving birth, a critical process that decreases the flow of blood to vessels that fed the placenta during pregnancy. Sometimes, particularly after difficult deliveries, the muscles are too tired to contract, and bleeding continues. Without speedy intervention, hemorrhage, which occurs in up to 10% of pregnancies, can quickly escalate, resulting in shock, an emergency hysterectomy or death.

Providers have tools to treat PPH, but when those fail, there’s the uterine balloon tamponade, a device that’s inserted into the uterus and filled with saline liquid, expanding to apply pressure to the uterine wall and stanch the bleeding. While studies show it to be reasonably effective—and certainly preferable to emergency surgery—it raised a question: Why would you expand the uterus when you wanted it to do the opposite? Why not just follow the body’s natural physiology? Norred and his peers began kicking around an alternative idea: a device that used gentle suction to get the uterus to contract.
Norred remembers the feedback from his professors and others as disbelieving and dismissive. Some feared the method would suck all the blood out of a woman’s body. But there were believers. Along the way, Norred had sought the advice of a family friend and ob/gyn, Dr. George Harper. Harper enlisted Dr. David Lagrew, a specialist in maternal fetal medicine who had recently led a California-wide hemorrhage task force. One gripe Lagrew heard repeatedly was about how cumbersome it could be to use a balloon tamponade—making the idea of an alternative approach especially appealing. The two doctors became advisors and co-inventors, working with Norred to conceptualize the device. Meanwhile Norred and his classmate, Davis Carlin, pressed on with a plan to make it a business.
The inventors found another champion in a 21-year-old go-getter named Jessie Becker Alexander, a business student who worked at Cal Poly’s innovation center, where Norred and Carlin had placed second in a competition and participated in a summer accelerator program. Becker Alexander says she “fell in love with what [the technology] could do for the world.” As Norred prepared to take a corporate job, she decided that if no one else was going to develop the device, she would. She became the CEO of the company, then named InPress Technologies, which she founded with Nathan Bair, a medical technology veteran who had recently begun advising startups at Cal Poly. Bair remembers thinking about the idea for the Jada device: “This is going to be a huge game changer.”
Given the number of women’s lives at stake, Bair was confident the startup would find some grant support. But prospective funders wanted to see proven technology first. So, it turned out, did venture capital.
Instead, Bair describes this as one of the company’s “wandering in the desert periods,” in which they scraped together cash from friends and found ways to be resourceful. On the advice of an ob/gyn, they used crushed silken tofu and pulpy orange juice to simulate uterine blood product; they also used another company’s lab animals for testing before they were euthanized.

That period also brought moments of serendipity. Early on, the founders discovered that the server at the coffee shop around the corner, Amelia Degenkolb, was a biomedical engineer. Degenkolb’s graduate thesis had focused on treating inguinal hemorrhage, or bleeding from the groin area, a battlefield injury that has become common with the proliferation of land mines. Her job options had been limited to weapons companies, and so she chose coffee—until InPress recruited her to be their lead engineer.
Breakthroughs, but little funding
By 2014, the team was testing the Jada in its first-in-woman study, a 10-patient trial at a hospital in Jakarta, Indonesia. PPH was considered a national emergency in Indonesia, and the founders had connected with an eager physician-collaborator there.
Degenkolb trained hospital staff on the device, and she was present for the first case. She watched anxiously as the doctor turned the device on, tending to a woman who was losing torrents of blood. The bleeding stopped almost instantly, and in that pause, Degenkolb was seized with terror—what had happened?—until she saw the attending physician’s expression, which went “from panic to calm to ecstatic. He had the biggest smile,” she says. Through light touch of the patient’s abdomen, he could feel the transformation of the uterus—it had contracted.
In the Jakarta trial, Jada controlled bleeding quickly all 10 times. The study’s findings ran in Obstetrics & Gynecologyin 2016, generating excitement among practitioners. In other fields of medicine, that data might have been the cue for funders or companies to swoop in with capital or acquisition offers. But Alydia still wasn’t generating much interest from investors.
Those involved at the time attribute that struggle in part to the team’s inexperience and strategy, but also to the unspoken laws that govern women’s health. “I couldn’t wrap my brain around how few people were ready to fund a company doing this,” says Degenkolb. She describes the business case for Jada as “dummy economics”: “If you stop someone from hemorrhaging, if you stop one blood transfusion, you've saved massive amounts of money.” But she attended pitch meetings with Becker Alexander and Bair, and she could sense when they’d lost the room (typically, of men): “As soon as we would say the word ‘uterus,’ you would just see their eyes glaze over and they’d disconnect.”
In fact, the InPress team’s biggest coup may have been landing a meeting with Merck for Mothers, the $650 million philanthropic arm of the drug company dedicated to improving maternal health worldwide. From early on, they'd seen Merck for Mothers as a natural ally for its mission to treat PPH in low- and middle-income countries. In what seems a common strategy among young, scrappy entrepreneurs—they’d invented a trip to New York and finagled some time on the organization’s calendar. It went well—“these are our people,” Bair remembers thinking—and a grant followed.
Grant money only goes so far, however, and InPress, which had taken residence at Fogarty Innovation, a Mountain View-based medtech incubator, was almost out of money and appeared to be approaching a dead end.
But at Fogarty, InPress also found crucial support, along with a new leader: Anne Morrissey, an industry veteran who had co-founded and sold device startups and worked in the women’s health space. At this precarious moment, Becker Alexander saw the best way to move the company forward was to put it in Morrissey’s more experienced hands.
Morrissey has three children, but until she met the InPress team she hadn’t thought much about PPH. From the outset, they’d discussed the complication as a “developing world problem” (indeed, that’s where most PPH-related deaths occur). But in talking with friends about the company, she’d inevitably learn about experiences of PPH within her own peer group, traumatic stories of women who, rather than spending their first hours with their newborns, were rushed off to receive emergency blood transfusions and found themselves packed with gauze, and tilted awkwardly in Trendelenberg position with their feet elevated above their heads.

By some accounts, that was a trajectory-shifting insight for InPress. It turned out that the complications associated with PPH were an almost invisible issue. Many ob/gyns would tell the team that PPH wasn’t a problem in the U,.S. because deaths were few. (PPH was the cause of 11% of America’s maternal deaths between 2016-18; as with other examples of America’s woeful maternal health statistics, Black women have a significantly higher risk of the worst outcomes.)
But Morrissey and her team realized that while PPH-driven mortality was low in the U.S., the complication still affected up to 360,000 women every year. Yes, America’s better health care resources prevented deaths, but the health and financial impacts associated with PPH, like longer hospital stays and severe morbidities, were still substantial and widespread. Cases of PPH are being reported with increasing frequency, a phenomenon linked to higher C-section rates and more mothers at risk due to health issues and other factors. According to Premier, a company that works with 60% of the nation’s health systems, deliveries that involve a hemorrhage cost $10,337 on average, 45% more than those that don’t.
The team recalibrated its pitch, leaning into the idea that the Jada device could be an important tool for the U.S. market, too. (Around this time, the company also got a new name, Alydia.) With this strategy, the company at last got its first institutional investor, the impact-oriented Global Health Investment Fund, which critically stepped in with capital needed for its trial in 2018. And the team's new message would help pave the way for a much bigger deal.
Organon: A women's health 'reboot'
Organon isn’t actually a new company, but a century-old reboot. Founded in 1923, the original company was started in a slaughterhouse in Oss, in the Netherlands, where an endocrinologist started experimenting with animal glands and hormone extraction. Over the years, the company became a reproductive health powerhouse, an innovator in at-home pregnancy tests, contraceptives, fertility drugs, and hormone replacement therapy. In 2006, those products generated $1.3 billion in revenue as scientists pursued innovations like male birth control and endometriosis meds.
Over the past 16 years, Organon was acquired by one American drug company and then sold to another, Merck. The important takeaway is that when Organon spun off from Merck in 2021, its women’s health portfolio was almost identical—essentially the same products, an additional $300 million in sales—to what it had in 2006. In the interim, its women’s health R&D had been shut down, with those dollars redirected to develop another of the assets Organon brought to Merck: the blockbuster cancer drug, Keytruda.
Merck’s calculation in deprioritizing women’s health research is one that other pharma companies have made as they’ve reoriented their portfolios around higher-priced specialty drugs. “All the money is chasing oncology,” acknowledges Organon’s Milligan. “And when it chases oncology, it walks away from other areas of therapeutic interest.”
The industry's embrace of specialty drugs also signaled a pivot away from “primary care” categories, women’s health included, that required large sales forces, says Gerberry, the Bank of America analyst. He adds that the incentive to invest in innovation in traditional women’s health products, like birth control, has declined with those products’ genericization, since payers usually require the use of low-cost generics rather than more expensive new products.
The field's historical baggage and safety scandals, meanwhile, have scared some companies away from taking bigger swings at developing treatments for conditions like uterine fibroids and preterm labor, where treatments are lacking. And many in the industry say U.S. regulators in women’s health are conservative and more demanding in terms of safety and efficacy data from clinical trials. They appreciate the FDA’s rigor, but say the effects make the development process even more expensive, and the path to market even more challenging. “You have to be a bit courageous,” explains Milligan. “Because you can't go into women's health if you are not going to go into those higher-risk areas…How can you be a women's health company and just sit and do contraceptives?”
While its ambitions (and deals) are real, Organon’s own women’s health branding is currently somewhat aspirational. Just a quarter of its $6.3 billion in annual revenue currently comes from that space; the majority of sales are generated by its “Established Brands” portfolio, which include dozens of Merck’s off-patent medicines, such as Nasonex and Propecia, that remain popular and important internationally. The company also has a third division—the one that seems to most excite those analysts—that will commercialize biosimilar drugs, essentially copies of biologic medicines like the blockbuster Humira, when they go off-patent.
This motley package of businesses began taking shape in 2019 when Merck was looking to simplify its portfolio, and Ali, a longtime executive there, was tasked by Merck’s then-CEO Ken Frazier with figuring out a way to do it. Ali didn’t set out to create a women’s health company, but it’s where he saw potential.
Merck’s women’s health business, though dormant, offered the foundation of products developed by the old Organon years ago. Ali imagined that many, with some added marketing muscle and more focused business attention, could be blockbusters. Chief among them was Nexplanon, a highly effective contraceptive implant that earned Merck $787 million in revenue in 2019 and offers patent exclusivity through at least 2027. There was also a neglected fertility business that continued to perform well due to growing demand worldwide.
Ali says he also felt cultural forces were shifting in favor of women’s health. Becoming the only publicly traded company focused on that field was a purpose he felt he could rally employees around, while the other divisions would generate a steady flow of cash to “give oxygen” to women’s health innovation.
Organon, was spun off as a purely commercial operation, created without its own drug pipeline or in-house R&D capabilities. Ali and Milligan say that’s a strength at this stage, allowing Organon to build out those features, both on its own and by acquiring the best assets from the growing women’s health field—in which there’s currently minimal competition.
View this interactive chart on Fortune.com
Analysts don’t seem wild about Organon’s approach. Leadership is frequently asked on earnings calls about the likelihood that the company will make a “transformational” acquisition. Gerberry muses, for example, on whether Organon might get into the “women’s health adjacent” aesthetics category (think Botox). But he notes that Organon doesn’t have much leeway on that front. Merck spun off the company with debt (“about four times levered,” Gerberry says), leaving it with limited financial capacity for the sort of deal that could quickly make it a high-growth company.
In any case, Milligan sees a bright future, comparing the women's health field’s now-percolating landscape to oncology’s a couple decades ago: “[It’s] a therapeutic area that had just a lack of investment, a lack of scientific progress, and all it took was a spark of a couple of successes,” she says. “I think about development as a relay race,” she adds, involving a chain of partners and investors to carry innovation along the way. She and others note that what’s really been lacking in women’s health are well-resourced companies like Organon that can take the baton and run, pulling the science through.
There are already signs that Organon is nurturing that ecosystem. Various Alydia alums, armed with their experience, are now working on other women’s health ventures. Degenkolb is developing a device targeting preterm labor, the leading cause of infant death and morbidity, which costs the U.S. $13.8 billion annually. Morrissey now leads May Health, a company developing a treatment for polycystic ovary syndrome. They’re among the entrepreneurs, investors, and executives whose work and purpose have been boosted, directly or indirectly, by Organon’s reemergence.
Jada reaches the market
And of course, Organon’s arrival was game-changing for Jada itself. The Alydia team already had the relationship it had built with Merck for Mothers, Organon's nonprofit corporate cousin. With Alydia’s sale to Organon—one of three companies that made a bid—the Jada now has the potential for a much wider reach, thanks to the acquirer’s global salesforce, which supplies products to 140 countries. The company acquired commitments to specific terms set by the Global Health Investment Fund, Alydia’s main investor, stipulating that it sell Jada in low- and middle-income countries at an affordable price and in a form that is viable in settings that lack electricity. (Alydia had already developed prototypes at the time of the sale.)
Organon says it’s working on that. For now, Jada is being rolled out across the U.S. and sold for about $1,000 per single-use device. It’s so far been used to treat more than 10,000 women in 800 hospitals and birth centers. The physicians and midwives Fortune contacted almost universally celebrated the device as an easy-to-use solution that has saved patients from emergency surgeries and hysterectomies, ICU stays, and more trauma in the delivery room.
“We’ve had tremendous success with it,” says Dr. Angela Bianco, professor and director of maternal fetal medicine for Mount Sinai in New York City, where they’ve used the device on more than 200 occasions. Bianco says the device has proven cost-effective, too; for her hospital, it’s only slightly more expensive than transfusing one unit of blood—and PPH patients usually need more blood than that, not to mention care for complications. At Northeast Georgia Medical Center, which sees many high-risk patients, they’ve used the device 34 times—it controlled the bleeding in 97% of cases. Jada has also cut down on blood transfusions there, which Dr. Keshma Saujani notes is significant, especially amidst an ongoing national blood shortage. “It came to us at the right time. We needed this,” she says.
Dr. Dilys Walker, a professor of Obstetrics, Gynecology and Reproductive Sciences at UCSF who serves on the World Health Organization’s PPH Guideline Development Group, describes herself as a “fan of Jada,” but also emphasizes that “PPH is a complex issue that requires a highly coordinated, efficient response to really be effective.” No individual tool is a silver bullet, Walker stresses: She’s leading a global research effort focused on how best to deploy new tools designed to address PPH, including Jada, more broadly in a way that avoids doing more harm than good.
Others, especially those who worked on Jada before Organon took over, express frustration that a lifesaving tool isn’t yet in use in parts of the world that could most benefit. Organon is in the early stages of its global rollout, launching in several countries, and pursuing regulatory approval in the EU. As for low- and middle-income countries, the company says it has its own global research effort underway: “It's a high priority need and one where it is critical to fully understand how Jada can work in these settings,” writes an Organon spokesperson.
Dr. Othiniel Musana, an ob/gyn at St. Francis Hospital Nsambya in Kampala, Uganda, first used the Jada in 2018 as part of Alydia’s trial work. In his career, he’s become all too familiar with “the period of terror” that comes with PPH, in a hospital that delivers more than 4,000 babies per year. He’s also the president of the Association of Obstetricians and Gynecologists of Uganda and chairs a committee that investigates the cause of every maternal death in the country. PPH accounts for 40% of the 6,000 that occur each year, a rate that remains stubbornly high due to shortages of personnel, medications, and blood.
All that made him enthusiastic to try the Jada. He was even more enthusiastic after using it. “Within about 30 seconds, the bleeding stopped,” he says. His hospital successfully treated multiple women as part of the study using Jada, but he hasn’t been able to get a device since. When Fortune mentioned its U.S. price, he noted that the sum is more than his monthly pay.
Still, Musana wishes the company had sought approval in Uganda immediately. “It was easy to use—very easy, actually,” he told Fortune, before adding, “The science around it was very basic. It’s something that I think should have been thought of much, much earlier.”
A grant from the NIHCM Foundation helped fund reporting for this story.